Does A Gas Have A Fixed Volume
Muz Play
Mar 31, 2025 · 6 min read
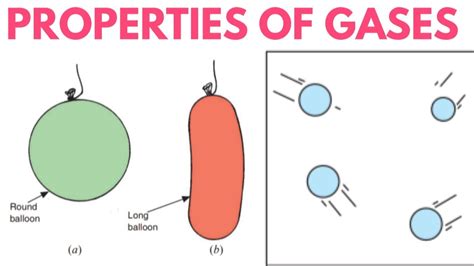
Table of Contents
Does a Gas Have a Fixed Volume? Understanding Gas Behavior
The question of whether a gas has a fixed volume is a fundamental concept in chemistry and physics. The simple answer is no, a gas does not have a fixed volume. Unlike solids and liquids, which maintain a relatively constant volume regardless of their container, gases are highly compressible and readily adapt to the shape and volume of their container. This characteristic stems from the unique properties of gas molecules and their interactions (or lack thereof). Understanding this behavior requires exploring the kinetic molecular theory of gases and the factors influencing gas volume.
The Kinetic Molecular Theory and Gas Volume
The kinetic molecular theory (KMT) provides a microscopic model explaining the macroscopic properties of gases. This theory posits several key assumptions that directly relate to the volume of a gas:
- Gases consist of tiny particles (atoms or molecules) that are in constant, random motion. These particles are in constant, chaotic movement, colliding with each other and the walls of their container.
- The volume of the gas particles themselves is negligible compared to the total volume of the gas. This means that the space occupied by the gas molecules is insignificant compared to the space between them. This assumption holds true for ideal gases, but real gases deviate from this ideal behavior at high pressures and low temperatures.
- There are no attractive or repulsive forces between gas particles. This is another idealization. Real gases exhibit intermolecular forces, which can affect their volume and behavior.
- Collisions between gas particles and container walls are elastic. This means that no energy is lost during collisions. The kinetic energy of the gas particles is conserved.
- The average kinetic energy of gas particles is directly proportional to the absolute temperature of the gas. This means that as temperature increases, the speed and energy of gas particles also increase.
These assumptions explain why gases expand to fill their containers. Because the volume of the gas particles is negligible and there are no significant intermolecular forces, the gas particles are free to move and spread out throughout the entire available space. Consequently, the volume of a gas is determined by the volume of its container.
Factors Affecting Gas Volume
Several factors influence the volume of a gas. These factors are interconnected and described by the ideal gas law (PV = nRT). Let's examine each of them in detail:
1. Pressure (P)
Pressure is defined as the force exerted per unit area. Increasing the pressure on a gas forces the gas particles closer together, reducing the volume. Conversely, decreasing the pressure allows the gas to expand, increasing its volume. This relationship is inversely proportional: as pressure increases, volume decreases, and vice versa, assuming constant temperature and amount of gas.
2. Volume (V)
The volume (V) is the space occupied by the gas. As mentioned earlier, the volume of a gas is not fixed; it adapts to the shape and size of its container. It's a dependent variable, influenced by pressure, temperature, and the amount of gas.
3. Temperature (T)
Temperature is a measure of the average kinetic energy of the gas particles. Increasing the temperature increases the kinetic energy of the particles, causing them to move faster and collide more frequently and forcefully with the container walls. This leads to an increase in pressure unless the volume is allowed to expand. If the pressure is kept constant, the volume of the gas will increase with increasing temperature. This relationship is directly proportional: as temperature increases, volume increases, assuming constant pressure and amount of gas. It's crucial to remember that temperature must be expressed in Kelvin (K) when using the ideal gas law.
4. Number of Moles (n)
The number of moles (n) represents the amount of gas present. One mole of any gas contains approximately 6.022 x 10<sup>23</sup> particles (Avogadro's number). Increasing the number of gas particles, while keeping pressure and temperature constant, leads to an increase in volume. This relationship is also directly proportional: as the number of moles increases, the volume increases.
Ideal Gas Law and Real Gas Behavior
The ideal gas law (PV = nRT) is a mathematical expression that relates the pressure, volume, temperature, and amount of an ideal gas. R is the ideal gas constant, a proportionality constant that depends on the units used for other variables. The ideal gas law is a powerful tool for predicting and calculating gas behavior under various conditions. However, it's essential to remember that the ideal gas law is a simplification. Real gases deviate from ideal behavior, particularly at high pressures and low temperatures.
Deviations from Ideal Gas Behavior
Real gases deviate from the ideal gas law because the assumptions of the KMT aren't perfectly accurate for all gases under all conditions. At high pressures, the volume occupied by the gas molecules becomes significant compared to the total volume, causing the volume to be larger than predicted by the ideal gas law. At low temperatures, intermolecular forces become more significant, leading to attractive forces between gas particles, reducing the volume.
Several equations of state, such as the van der Waals equation, attempt to account for these deviations by incorporating terms that correct for the volume of the gas particles and the intermolecular forces. These equations provide more accurate predictions of real gas behavior, especially under conditions far from ideal.
Applications and Real-World Examples
Understanding the non-fixed volume of gases is crucial in many scientific and engineering applications:
- Pneumatics and Hydraulics: The compressibility of gases is fundamental to pneumatic systems (using compressed air or gas) and hydraulic systems (using liquids). Understanding how gas volume changes with pressure is essential for designing efficient and safe systems.
- Meteorology: Weather patterns are influenced by changes in air pressure, temperature, and humidity, all of which affect the volume of gases in the atmosphere.
- Aerospace Engineering: The behavior of gases at high altitudes and low pressures is critical in designing aircraft and spacecraft. Understanding gas expansion and contraction is vital for ensuring safe and efficient operation.
- Chemical Engineering: Many industrial processes involve gases, and controlling their volume and pressure is essential for optimizing reaction yields and efficiency.
- Deep-Sea Diving: The compressibility of gases is a major concern for deep-sea divers. As divers descend, the pressure increases, causing the volume of gases in their bodies to decrease. This necessitates careful management of breathing gases to avoid decompression sickness.
Conclusion
In summary, a gas does not possess a fixed volume. Its volume is highly dependent on pressure, temperature, and the amount of gas present. The kinetic molecular theory provides a microscopic explanation for this behavior, and the ideal gas law offers a mathematical framework for predicting and calculating gas volume under various conditions. Although the ideal gas law provides a useful approximation, real gases deviate from ideal behavior, especially under extreme conditions. Understanding the factors affecting gas volume is crucial in numerous scientific, engineering, and industrial applications. Appreciating the dynamic and adaptable nature of gas volume is fundamental to comprehending the behavior of matter in various contexts.
Latest Posts
Latest Posts
-
Plant Cell In A Isotonic Solution
Apr 02, 2025
-
How To Tell Left And Right Ulna
Apr 02, 2025
-
What Is A Change Agent In Nursing
Apr 02, 2025
-
The Size Of A Cell Is Limited By The
Apr 02, 2025
-
Explain The Role Of Organisms In The Carbon Cycle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Does A Gas Have A Fixed Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
