Weak Acid Strong Base Titration Equivalence Point
Muz Play
Mar 18, 2025 · 7 min read
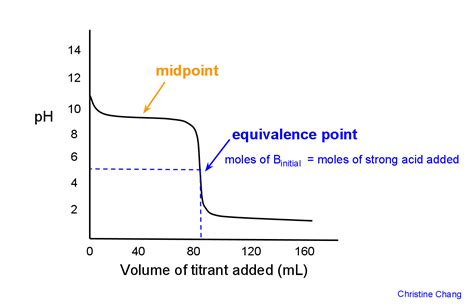
Table of Contents
Weak Acid-Strong Base Titration: Understanding the Equivalence Point
Titration is a fundamental analytical technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. One common type of titration involves the reaction between a weak acid and a strong base. Understanding the equivalence point in this type of titration is crucial for accurate analysis. This comprehensive guide will delve into the intricacies of weak acid-strong base titrations, focusing specifically on the equivalence point and the factors influencing it.
What is a Weak Acid-Strong Base Titration?
A weak acid-strong base titration involves the gradual addition of a strong base (e.g., NaOH, KOH) to a solution of a weak acid (e.g., acetic acid, CH₃COOH; formic acid, HCOOH). The reaction that occurs is a neutralization reaction, where the hydrogen ions (H⁺) from the weak acid react with the hydroxide ions (OH⁻) from the strong base to form water (H₂O). This reaction proceeds until all the weak acid is neutralized.
Key Characteristics:
- Weak Acid: Doesn't completely dissociate in water; only a small fraction of the acid molecules donate their protons. This results in a solution with a lower concentration of H⁺ ions compared to a strong acid of the same concentration.
- Strong Base: Completely dissociates in water, producing a high concentration of OH⁻ ions.
- Neutralization Reaction: H⁺ (from weak acid) + OH⁻ (from strong base) → H₂O
- Buffer Region: A significant characteristic of this titration is the presence of a buffer region before the equivalence point. This region is characterized by a relatively small change in pH upon the addition of small volumes of the strong base.
The Equivalence Point: Defining the Crucial Moment
The equivalence point is the point in the titration where the moles of the strong base added are stoichiometrically equal to the moles of the weak acid initially present. This means that all the weak acid molecules have reacted with the hydroxide ions from the strong base, leaving only the conjugate base of the weak acid and water in the solution. It's a critical point because it represents the complete neutralization of the weak acid.
Crucial Difference from the End Point:
It's important to differentiate the equivalence point from the end point. The equivalence point is a theoretical point determined by the stoichiometry of the reaction. The end point, on the other hand, is the point at which the indicator (if used) changes color, signaling the completion of the titration. While ideally, these two points coincide, in reality, there's usually a slight difference. This difference is the titration error.
Calculating the pH at the Equivalence Point
Determining the pH at the equivalence point for a weak acid-strong base titration requires understanding the equilibrium established by the conjugate base of the weak acid. After the equivalence point is reached, the solution contains only the conjugate base of the weak acid and water. The conjugate base reacts with water in a hydrolysis reaction:
A⁻ + H₂O ⇌ HA + OH⁻
where A⁻ represents the conjugate base. The pH is determined by the concentration of hydroxide ions (OH⁻) produced in this hydrolysis reaction.
Steps to Calculate the pH at the Equivalence Point:
-
Determine the moles of weak acid initially present: This is obtained from the initial volume and concentration of the weak acid solution.
-
Calculate the concentration of the conjugate base at the equivalence point: The moles of conjugate base formed are equal to the initial moles of weak acid. The volume at the equivalence point is the sum of the initial volume of weak acid and the volume of strong base added.
-
Determine the Kb of the conjugate base: The Kb is related to the Ka (acid dissociation constant) of the weak acid by the equation: Kb = Kw/Ka, where Kw is the ion product constant for water (1.0 x 10⁻¹⁴ at 25°C).
-
Use the Kb expression to calculate the hydroxide ion concentration: The Kb expression for the hydrolysis reaction is: Kb = [HA][OH⁻]/[A⁻]. Since [HA] = [OH⁻] at equilibrium, we can simplify this to: Kb = [OH⁻]²/ [A⁻]. Solving for [OH⁻] gives the hydroxide ion concentration.
-
Calculate the pOH: pOH = -log[OH⁻]
-
Calculate the pH: pH = 14 - pOH
Factors Affecting the pH at the Equivalence Point
Several factors influence the pH at the equivalence point of a weak acid-strong base titration:
-
The Ka of the weak acid: A weaker acid (smaller Ka) will have a conjugate base that is a stronger base, resulting in a higher pH at the equivalence point. Conversely, a stronger weak acid will have a lower pH at the equivalence point.
-
The concentration of the weak acid: A more concentrated solution of the weak acid will result in a higher concentration of the conjugate base at the equivalence point, leading to a slightly higher pH.
-
Temperature: The Kw value changes with temperature, influencing the pH calculations.
-
Ionic Strength: The presence of other ions in the solution can affect the activity coefficients of the ions involved, slightly altering the pH. However, this effect is generally small for dilute solutions.
The Titration Curve: A Visual Representation
The titration curve is a graph that plots the pH of the solution against the volume of strong base added. It provides a visual representation of the titration process, showing the different regions of the titration, including the buffer region and the equivalence point.
Key Features of the Titration Curve:
-
Initial pH: The pH of the weak acid solution before the addition of any strong base. This pH will be slightly acidic.
-
Buffer Region: A region of relatively slow pH change, where the solution acts as a buffer, resisting changes in pH. This region exists before the equivalence point.
-
Equivalence Point: The point where the moles of strong base added equal the moles of weak acid initially present. The pH at this point will be greater than 7 (basic) due to the hydrolysis of the conjugate base.
-
Post-Equivalence Point: After the equivalence point, the pH increases rapidly with the addition of small volumes of strong base. The solution becomes increasingly basic.
The shape of the titration curve provides valuable information about the strength of the weak acid and its concentration.
Applications of Weak Acid-Strong Base Titrations
Weak acid-strong base titrations are used in various analytical applications, including:
-
Determining the concentration of weak acids: This is a primary application of the technique. By titrating a weak acid with a strong base of known concentration, the concentration of the weak acid can be determined accurately.
-
Determining the Ka of a weak acid: The shape of the titration curve and the pH at the half-equivalence point can be used to determine the Ka of the weak acid.
-
Analyzing pharmaceutical compounds: Many drugs are weak acids or bases, and titration is used to analyze their purity and concentration.
-
Environmental monitoring: Titration is used to measure the acidity or alkalinity of water samples, providing information about water quality.
-
Food and beverage industry: Titration is used in various applications, such as determining the acidity of fruit juices or vinegar.
Choosing the Right Indicator
The choice of indicator for a weak acid-strong base titration is important to ensure accurate results. The indicator should have a pKa value close to the pH at the equivalence point. Phenolphthalein is a commonly used indicator for this type of titration because its color change occurs in the pH range suitable for detecting the equivalence point. However, the specific indicator choice depends on the nature of the weak acid and its Ka value.
Conclusion: Mastering the Equivalence Point for Accurate Results
Understanding the equivalence point in a weak acid-strong base titration is fundamental to accurately determining the concentration of the weak acid. By carefully considering the stoichiometry of the reaction, the equilibrium involved, and the factors affecting the pH at the equivalence point, one can perform precise titrations with reliable results. The titration curve, along with the appropriate indicator, provides a powerful tool for obtaining valuable analytical data in various scientific and industrial applications. Mastering this technique enables accurate quantitative analysis crucial for many fields.
Latest Posts
Latest Posts
-
How To Find Bond Dissociation Energy
Mar 18, 2025
-
Where Does Mrna Go After It Leaves The Nucleus
Mar 18, 2025
-
What Is The Difference Between Primary And Secondary Growth
Mar 18, 2025
-
Work Done By An Electric Field
Mar 18, 2025
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Weak Acid Strong Base Titration Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
