What Are Rows Of The Periodic Table Called
Muz Play
Mar 21, 2025 · 6 min read
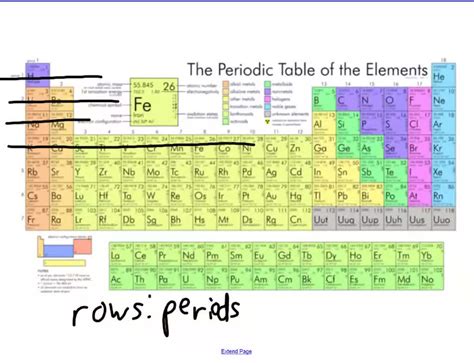
Table of Contents
What Are Rows of the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While most are familiar with the columns, called groups or families, understanding the rows, known as periods, is equally crucial for comprehending the intricacies of atomic structure and chemical behavior. This comprehensive article will delve into the meaning of "periods" in the periodic table, explore their characteristics, and discuss their significance in various chemical and physical applications.
Understanding Periods: A Horizontal Journey Through Atomic Structure
The rows of the periodic table, the periods, represent elements with the same number of electron shells. Each period begins with an element possessing a single electron in a new electron shell and ends with a noble gas, an element with a completely filled valence shell. This fundamental structural similarity significantly influences the chemical properties of elements within a given period.
Period 1: The Simplest Beginnings
The first period is the shortest, containing only two elements: hydrogen (H) and helium (He). These elements possess only one electron shell, making them distinct from the elements in subsequent periods. Hydrogen, with its single electron, is unique in its ability to act as both an oxidizing and reducing agent, while helium, with a full valence shell, is an extremely inert noble gas. This stark contrast highlights the unique characteristics of the first period.
Period 2 and 3: The Emergence of Shell Structure Complexity
Periods 2 and 3 each contain eight elements, a number dictated by the maximum number of electrons that can occupy the s and p subshells of a principal energy level. These periods witness the introduction of increasingly complex atomic structures and a broadening range of chemical behaviors. For instance, Period 2 includes the transition from highly reactive alkali metals (like lithium and sodium) to increasingly electronegative nonmetals (like oxygen and fluorine), culminating in the inert noble gas neon. This trend of increasing electronegativity across a period is a hallmark of periodic table organization.
Periods 4 and 5: The Introduction of d-block Elements
Periods 4 and 5 are longer than previous periods, incorporating the d-block elements, also known as transition metals. These elements have electrons filling the d orbitals, leading to a greater diversity of oxidation states and complex coordination chemistry. The transition metals are known for their vibrant colors, catalytic activity, and roles in numerous biological processes and industrial applications. The addition of the d-block significantly increases the complexity of chemical behavior within these periods. The longer periods also showcase more nuanced variations in metallic character, electronegativity and other properties.
Periods 6 and 7: The Inclusion of f-block Elements and Radioactive Elements
Periods 6 and 7 are the longest periods and include the f-block elements, also known as inner transition metals (lanthanides and actinides). These elements have electrons filling the f orbitals, adding another layer of complexity to their atomic structure and chemical behavior. The actinides, in particular, are notable for their radioactivity, making them crucial in nuclear technology and research. The inclusion of the f-block elements represents the pinnacle of atomic structure complexity in the periodic table, adding a significant dimension to the understanding of chemical behavior.
Many elements within these periods also showcase significant radioactivity, further expanding the understanding of atomic behavior beyond simple chemical reactivity. The inclusion of radioactive elements introduces elements with vastly differing half-lives, making them essential in areas such as nuclear medicine and energy production.
The Significance of Periodicity in Chemical Properties
The arrangement of elements into periods reflects a fundamental periodicity in their chemical and physical properties. This periodicity arises from the regular recurrence of similar electron configurations in the outermost electron shell (valence shell). The valence electrons primarily determine the chemical behavior of an element.
Trends Across a Period: Electronegativity, Ionization Energy, and Atomic Radius
Several key trends in properties are observed across a period:
-
Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period from left to right. This is due to the increasing nuclear charge and the decrease in atomic radius, leading to a stronger pull on electrons.
-
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This trend is also due to the increasing nuclear charge and decreasing atomic radius, making it more difficult to remove an electron.
-
Atomic Radius: Atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. This decrease is a consequence of the increasing nuclear charge, which pulls the electrons closer to the nucleus.
Understanding these trends is crucial for predicting the reactivity and chemical behavior of elements within a particular period.
Trends Down a Group: A Comparison with Periodicity
While periods show trends across a row, groups (columns) reveal trends down the column. These trends are predominantly determined by the similar electronic configuration within the valence shell. While periodic trends illustrate the changes across a period, group trends explain the similarities down a column, demonstrating the complementary nature of these organizational features of the periodic table.
Applications and Significance of Periodicity
The understanding of periods and their associated trends has far-reaching implications across diverse scientific fields:
Chemistry: Predicting Reactivity and Bonding
The periodic table's organization allows chemists to predict the reactivity of elements based on their position. The alkali metals (Group 1) are highly reactive due to their single valence electron, readily losing it to form a +1 ion. The halogens (Group 17) are also highly reactive, readily gaining an electron to form a -1 ion. The noble gases (Group 18), with their complete valence shells, are generally inert. This predictive power is crucial in designing and understanding chemical reactions.
Materials Science: Designing Novel Materials
The periodic table serves as a guide for materials scientists in designing novel materials with specific properties. The knowledge of how properties vary across periods allows them to select elements that will produce materials with desired characteristics, such as strength, conductivity, or magnetism. This understanding is vital in creating materials for electronics, construction, and aerospace applications.
Biology: Understanding Biomolecules and Biological Processes
The periodic table plays a crucial role in understanding biological processes. Many essential biomolecules, such as proteins and nucleic acids, incorporate elements from specific periods. The chemical behavior of these elements is essential for the functioning of these biomolecules and, consequently, for life itself.
Nuclear Chemistry: Understanding Radioactive Decay and Nuclear Reactions
The periodic table's inclusion of radioactive elements (especially in Periods 6 and 7) is paramount for nuclear chemistry. Understanding the decay pathways and properties of these elements is crucial for applications in nuclear medicine, energy production, and environmental monitoring. The long periods encapsulate a wealth of radioactive isotopes critical to these areas.
Conclusion: Periods as a Foundation for Chemical Understanding
In conclusion, understanding the rows of the periodic table—the periods—is essential for comprehending the fundamental principles of chemistry. The regular recurrence of similar electron configurations within each period leads to a predictable pattern in the chemical and physical properties of elements. This periodicity is a cornerstone of chemical understanding, crucial in predicting reactivity, designing new materials, and understanding biological processes. From the simplest elements in Period 1 to the complex radioactive elements in Periods 6 and 7, the periods provide a structured framework for exploring the fascinating world of chemistry and its diverse applications. The periodic table's arrangement, far from being a mere organizational tool, is a powerful representation of the underlying order and predictability of the chemical elements and their interactions.
Latest Posts
Latest Posts
-
Standard Error For Difference In Means
Mar 22, 2025
-
Definition Of Reference Group In Sociology
Mar 22, 2025
-
The Study Of The Geographical Distribution Of Organisms Is Called
Mar 22, 2025
-
Lewis Dot Structures For Polyatomic Ions
Mar 22, 2025
-
Is Tap Water A Homogeneous Mixture
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Are Rows Of The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
