What Are The Boiling And Freezing Points Of Water
Muz Play
Apr 06, 2025 · 5 min read
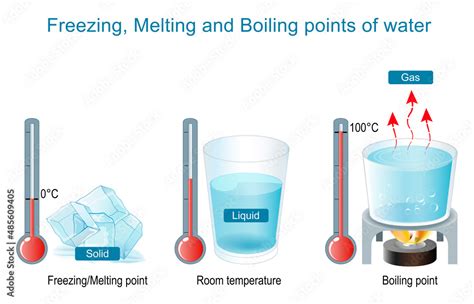
Table of Contents
What Are the Boiling and Freezing Points of Water? A Deep Dive
Water, the elixir of life, is a substance so ubiquitous that we often take its fundamental properties for granted. Yet, the seemingly simple question, "What are the boiling and freezing points of water?" opens a fascinating window into the world of thermodynamics, phase transitions, and the unique characteristics of this essential molecule. This article will delve deep into these points, exploring not just the numbers but the science behind them, influencing factors, and practical applications.
Understanding Phase Transitions: From Solid to Liquid to Gas
Before we dive into the specific temperatures, let's establish a foundational understanding of phase transitions. Water, like all matter, exists in three primary phases: solid (ice), liquid (water), and gas (water vapor or steam). These phases are determined by the energy level of the water molecules.
-
Freezing: When water transitions from a liquid to a solid (freezing), its molecules lose kinetic energy, slowing down and becoming less mobile. This reduced energy allows the molecules to form a structured crystalline lattice, characteristic of ice.
-
Melting: The reverse process, melting, occurs when ice absorbs heat energy, increasing the kinetic energy of its molecules. This increased energy overcomes the intermolecular forces holding the crystal lattice together, causing the ice to transition into liquid water.
-
Boiling: When liquid water is heated, its molecules absorb energy and move faster. Eventually, this energy overcomes the attractive forces between water molecules, allowing them to escape the liquid phase and transition into a gas (boiling). This occurs at the boiling point.
-
Condensation: The opposite of boiling, condensation occurs when water vapor loses energy and transitions back into a liquid.
These phase transitions are crucial for countless natural processes and industrial applications.
The Boiling Point of Water: More Than Just 100°C
The boiling point of water is commonly cited as 100 degrees Celsius (212 degrees Fahrenheit) at standard atmospheric pressure (1 atmosphere or 101.325 kPa). However, this is a simplification. The boiling point is actually dependent on pressure.
The Impact of Pressure on Boiling Point
At higher altitudes, where atmospheric pressure is lower, the boiling point of water decreases. This is because the reduced pressure requires less energy for water molecules to overcome the attractive forces and escape into the gaseous phase. Conversely, at higher pressures, the boiling point increases because more energy is needed to overcome the stronger forces holding the molecules together.
Practical Applications of Pressure's Influence
This relationship between pressure and boiling point has significant practical implications:
-
Cooking at High Altitudes: At high altitudes, water boils at a lower temperature, meaning food takes longer to cook. Pressure cookers counteract this by increasing the internal pressure, raising the boiling point and speeding up the cooking process.
-
Steam Sterilization: The high temperatures achievable through steam sterilization under pressure are crucial in medical and industrial settings for effective disinfection.
-
Power Generation: Steam turbines in power plants rely on high-pressure steam to generate electricity.
The Freezing Point of Water: Why 0°C?
The freezing point of water, like its boiling point, isn't always a fixed value. While it's commonly stated as 0 degrees Celsius (32 degrees Fahrenheit) at standard atmospheric pressure, it can be affected by several factors.
Factors Affecting the Freezing Point
-
Pressure: While the effect of pressure on the freezing point of water is less pronounced than on the boiling point, increased pressure slightly lowers the freezing point. This is why ice skates can glide on ice; the pressure from the skates slightly melts the ice, creating a lubricating layer.
-
Dissolved Substances: The presence of dissolved substances (solutes) in water, such as salt, lowers the freezing point. This phenomenon, known as freezing point depression, is why salt is used to de-ice roads in winter. The salt dissolves in the thin layer of water on the road, lowering its freezing point and preventing ice formation.
-
Impurities: Other impurities in the water, even in small quantities, can subtly affect the freezing point.
Practical Applications of Freezing Point Depression
-
De-icing: As mentioned above, the use of salt to lower the freezing point of water is a common application.
-
Antifreeze: Antifreeze solutions used in car radiators contain chemicals that lower the freezing point of water, preventing damage to the engine during cold weather.
-
Food Preservation: Freezing food relies on lowering the temperature to inhibit the growth of microorganisms and slow down enzymatic reactions.
The Uniqueness of Water's Properties
Water's boiling and freezing points are not just arbitrary numbers; they are consequences of the unique properties of the water molecule (H₂O). The strong hydrogen bonds between water molecules contribute significantly to its relatively high boiling point compared to other similar-sized molecules. These hydrogen bonds require a substantial amount of energy to break, leading to a higher boiling point. The crystalline structure formed by ice also contributes to its unique properties.
Beyond the Basics: Further Exploration
Understanding the boiling and freezing points of water goes beyond simply knowing the numbers. It involves grasping the underlying principles of thermodynamics, phase transitions, and the impact of external factors like pressure and dissolved substances.
Here are some areas for further exploration:
-
Triple Point of Water: This is the unique temperature and pressure at which water can coexist in all three phases—solid, liquid, and gas—in equilibrium.
-
Critical Point of Water: This point represents the temperature and pressure above which the distinction between liquid and gas phases disappears.
-
Supercooled Water: This is a metastable state of water where it remains liquid below its normal freezing point.
-
The Anomalous Behavior of Water: Water exhibits several unique behaviors, such as its maximum density at 4°C, that deviate from the typical trends observed in other substances.
Conclusion: The Significance of Understanding Water's Phase Transitions
The seemingly simple question of water's boiling and freezing points opens a door to a world of fascinating scientific principles and practical applications. Understanding these points, and the factors that influence them, is critical in numerous fields, from cooking and engineering to meteorology and environmental science. The unique properties of water, stemming from its molecular structure and the strong hydrogen bonds between its molecules, make it a truly exceptional substance, essential for life as we know it. This deep dive into the boiling and freezing points of water hopefully provides a more comprehensive understanding of this fundamental aspect of our world. Further research into the more nuanced aspects of water's behavior will undoubtedly reveal even more fascinating insights into this remarkable molecule.
Latest Posts
Latest Posts
-
The Active Site Is Located On The Substrate
Apr 08, 2025
-
The Halogens Are Found In Group
Apr 08, 2025
-
Calculating Heat Of Reaction From Bomb Calorimetry Data
Apr 08, 2025
-
What Is The Iupac Name For This Alkane
Apr 08, 2025
-
How To Interpret Post Hoc Test
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Are The Boiling And Freezing Points Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.