What Are The Horizontal Rows In The Periodic Table Called
Muz Play
Mar 18, 2025 · 7 min read
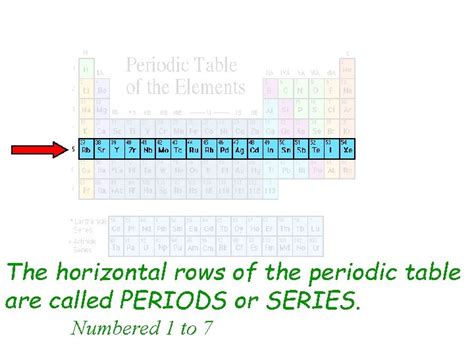
Table of Contents
What are the Horizontal Rows in the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While many are familiar with the vertical columns, known as groups or families, the horizontal rows also hold significant meaning. These horizontal rows are called periods. Understanding periods is crucial to grasping the trends and patterns in element behavior, reactivity, and properties. This comprehensive guide will delve into the intricacies of periods, exploring their structure, significance, and the fascinating relationships between elements within each row.
Understanding Periods: A Horizontal Journey Through Atomic Structure
The periodic table's arrangement isn't arbitrary; it's a reflection of the underlying quantum mechanics governing atomic structure. Each period represents an energy level, or shell, within an atom. As we move across a period, we add one proton and one electron, systematically filling the electron shells. This systematic addition leads to predictable changes in atomic size, ionization energy, and electronegativity, forming the basis of periodic trends.
Period 1: The Simplest Beginnings
Period 1, the shortest period, contains only two elements: hydrogen (H) and helium (He). Both elements fill the first electron shell (n=1), which can hold a maximum of two electrons. Hydrogen, with one electron, is highly reactive, while helium, with a full electron shell, is remarkably inert, showcasing the impact of electron configuration on chemical behavior. This is the foundation for understanding the principles of the octet rule and electron stability.
Period 2 and 3: Expanding Shells and Increasing Complexity
Periods 2 and 3, both containing eight elements, represent the filling of the second and third electron shells, respectively. These shells contain s and p sublevels, adding to the complexity of electron arrangements. The elements in these periods demonstrate a wider range of properties and reactivity compared to Period 1. For instance, Period 2 includes lithium (Li), an alkali metal, and fluorine (F), a highly reactive halogen, highlighting the significant differences that emerge as we progress across the period. The trend of increasing electronegativity across the period is clearly visible in these rows.
Period 4 and 5: The Introduction of d-Block Elements
Periods 4 and 5 are significantly longer than the preceding periods due to the introduction of the d sublevel. The d sublevel can accommodate up to ten electrons, resulting in the inclusion of the transition metals. These transition metals exhibit variable oxidation states, meaning they can lose different numbers of electrons in chemical reactions. This property contributes to their diverse chemical behavior and catalytic capabilities, making them essential in numerous industrial applications and biological processes. The transition metals also exhibit characteristic color changes in solution, further highlighting their unique properties. The properties of these elements are heavily influenced by the filling of the d orbitals and the shielding effect of inner electrons.
Period 6 and 7: f-Block Elements and the Lanthanides and Actinides
Periods 6 and 7 are the longest periods, showcasing the inclusion of the f sublevel. The f sublevel can accommodate up to fourteen electrons, leading to the inclusion of the lanthanides (rare earth elements) and actinides. These elements are mostly radioactive and found towards the bottom of the periodic table, often separated for clarity. They exhibit unique properties due to the complex interactions within their f electron shells. The actinides are particularly noteworthy due to their radioactivity and roles in nuclear energy and weaponry.
The Significance of Periodicity in Chemical Properties
The horizontal arrangement of the periodic table reveals crucial trends in chemical properties as we move across a period. Understanding these trends provides insights into how elements react and interact with each other.
Atomic Radius: A Decreasing Trend
Generally, atomic radius decreases as we move from left to right across a period. This is because the number of protons increases, attracting the electrons more strongly, thereby pulling the electron cloud closer to the nucleus. The addition of electrons to the same shell does not counteract the increasing nuclear charge significantly.
Ionization Energy: An Increasing Trend
Ionization energy, the energy required to remove an electron from an atom, generally increases as we move across a period. This is a direct consequence of the increasing nuclear charge and the decreasing atomic radius. The stronger attraction between the nucleus and electrons makes it more difficult to remove an electron.
Electronegativity: The Pull on Electrons
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also generally increases as we move across a period. This increase is directly linked to the increasing nuclear charge and decreasing atomic radius. The stronger attraction for electrons makes the atom more likely to attract electrons from other atoms in a bond.
Metallic Character: From Left to Right
Metallic character, indicating properties such as conductivity and malleability, generally decreases as we move across a period. This is because the electrons are held more tightly to the nucleus across a period, reducing the ability of atoms to donate electrons and form metallic bonds.
Periodicity and its Applications
The understanding of periods and their related periodic trends finds widespread applications in various fields:
- Predicting chemical reactions: Knowledge of periodic trends allows chemists to predict the reactivity of elements and anticipate the products of chemical reactions.
- Material science: The properties of elements within a period guide the design and synthesis of novel materials with specific characteristics, like strength, conductivity, or reactivity.
- Biological systems: Understanding the role of trace elements within specific periods helps us to analyze biological processes and comprehend the effects of essential nutrients.
- Environmental science: The knowledge of how different elements interact, influenced by their periodic position, is vital in environmental monitoring and remediation efforts.
Beyond the Basics: Deeper Insights into Periodicity
The concept of periods extends beyond simply organizing elements based on electron shells. It also provides a framework for understanding other fundamental chemical concepts.
Valence Electrons and Chemical Bonding
The number of valence electrons, electrons in the outermost shell, determines the chemical reactivity of an element and its ability to form chemical bonds. Elements within the same group have the same number of valence electrons, explaining their similar chemical behavior. However, the valence electrons also play a crucial role in the properties of elements within the same period. The increasing number of protons across a period influences how strongly the valence electrons are held, impacting bonding behavior.
Effective Nuclear Charge and Shielding Effect
The effective nuclear charge, the net positive charge experienced by an electron, influences the properties of an element. The shielding effect, caused by inner electrons, reduces the attraction between the nucleus and outer electrons. Both effective nuclear charge and shielding effect vary as we move across a period, contributing to the observed trends in atomic size, ionization energy, and electronegativity.
Electron Configurations and Periodicity
Electron configurations, the arrangement of electrons in an atom's shells and subshells, are directly related to the position of an element in the periodic table. Elements in the same period have the same number of electron shells, but the number of electrons in the outermost shell changes as we move across a period. The subtle variations in electron configurations account for the differences in chemical properties among elements in the same period.
Conclusion: The Enduring Importance of Periods
The horizontal rows, or periods, in the periodic table are far more than a simple organizational feature. They represent a fundamental aspect of atomic structure, directly influencing the chemical and physical properties of elements. Understanding periods provides a framework for predicting chemical behavior, designing new materials, and interpreting biological and environmental processes. As we delve deeper into the nuances of atomic structure and quantum mechanics, the significance of periods in the periodic table becomes increasingly clear, highlighting their enduring importance in chemistry and beyond. The systematic arrangement and predictable trends within periods are a testament to the elegance and power of the periodic table, a timeless tool for understanding the building blocks of matter.
Latest Posts
Latest Posts
-
How To Compute Cost Per Equivalent Unit
Mar 18, 2025
-
What Are The Physical Properties Of A Metal
Mar 18, 2025
-
Why Is The Force Subscript Not Written In The Us
Mar 18, 2025
-
A Relationship In Which Both Organisms Benefit
Mar 18, 2025
-
If One Of The Reactants In A Reaction Is
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Are The Horizontal Rows In The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
