What Are The Most Reactive Metals On The Periodic Table
Muz Play
Mar 29, 2025 · 6 min read
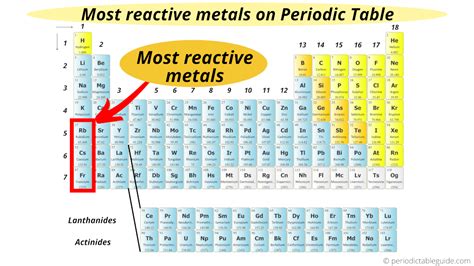
Table of Contents
What Are the Most Reactive Metals on the Periodic Table?
The periodic table, a beautifully organized chart of elements, reveals much more than just atomic weights and numbers. It's a roadmap to understanding the fundamental properties of matter, and reactivity is a key characteristic that dictates how elements interact with their environment. Among these elements, metals stand out for their propensity to lose electrons and form ions, a process that drives their reactivity. This article delves into the fascinating world of reactive metals, exploring which ones are the most eager to participate in chemical reactions and the reasons behind their behavior.
Understanding Reactivity: The Role of Electrons
Before diving into specific elements, it's crucial to grasp the fundamental concept of reactivity in metals. Reactivity is essentially the tendency of a substance to undergo a chemical change. In the case of metals, this change is primarily driven by their electronic configuration. Metals are characterized by their relatively low electronegativity, meaning they have a weaker hold on their valence electrons (the electrons in the outermost shell). These loosely held electrons are easily lost, leading to the formation of positively charged ions (cations). The easier a metal loses its electrons, the more reactive it is.
This electron loss is often accompanied by the release of energy, making many reactions of highly reactive metals exothermic (heat-releasing). The stronger the attraction between the metal's electrons and other atoms or molecules (like oxygen or chlorine), the more vigorous the reaction will be. This is why reactive metals are often stored under inert conditions, such as in oil or argon gas, to prevent uncontrolled reactions with air and moisture.
The Alkali Metals: The Champions of Reactivity
The alkali metals, located in Group 1 of the periodic table (the first column), are renowned for their exceptional reactivity. This group includes lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). They all have a single valence electron, which is very easily lost. This single electron makes them highly reactive, readily forming +1 ions.
Lithium (Li):
While the least reactive of the alkali metals, lithium still reacts vigorously with water, producing hydrogen gas and lithium hydroxide. The reaction is less dramatic than that of heavier alkali metals, but it's still noticeable. Lithium is also known for its use in batteries, taking advantage of its ability to readily donate its electron.
Sodium (Na):
Sodium is far more reactive than lithium. It reacts violently with water, producing a significant amount of heat and hydrogen gas. The reaction is often accompanied by a bright flame. Sodium's reactivity is a key factor in its various applications, from street lighting (sodium vapor lamps) to the production of various chemicals.
Potassium (K):
Potassium surpasses sodium in reactivity. Its reaction with water is even more vigorous and exothermic, often leading to ignition of the hydrogen gas produced. The reaction is so energetic that it should only be performed by trained professionals with appropriate safety precautions.
Rubidium (Rb) and Cesium (Cs):
Moving down the group, rubidium and cesium exhibit even greater reactivity. They react explosively with water, and even exposure to air can lead to spontaneous combustion. These metals require careful handling and storage under inert conditions.
Francium (Fr):
Francium, the last member of the alkali metal group, is the most reactive metal on the periodic table. However, due to its extreme rarity and radioactivity, its reactivity is largely theoretical and based on its position in the periodic table. It's highly unstable and quickly decays into other elements.
The Alkaline Earth Metals: A Step Down, But Still Reactive
The alkaline earth metals, found in Group 2 of the periodic table, are also highly reactive, though generally less so than the alkali metals. This group includes beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They each have two valence electrons, which can be lost to form +2 ions.
While not as explosive as the alkali metals, alkaline earth metals still react readily with water and oxygen. Magnesium, for example, burns brightly in air, producing a dazzling white light, a property used in flares and fireworks. Calcium, crucial for biological systems, reacts less vigorously with water but still demonstrates its metallic character through this reaction. Beryllium is an exception, exhibiting significantly lower reactivity than the other members of this group due to its small size and high ionization energy.
Other Highly Reactive Metals
Beyond the alkali and alkaline earth metals, several other metals exhibit significant reactivity:
-
Aluminum (Al): Aluminum is surprisingly reactive, readily forming a protective oxide layer that prevents further reaction. This layer protects the metal from corrosion. However, finely divided aluminum powder or aluminum foil can react vigorously under certain conditions.
-
Zinc (Zn): Zinc is a moderately reactive metal, readily reacting with acids and bases. It is commonly used in galvanization to protect iron from rust.
-
Iron (Fe): Iron's reactivity is lower than that of the alkali and alkaline earth metals, but it still readily reacts with oxygen and water, forming rust. This corrosion is a significant issue in many applications.
Factors Affecting Reactivity
Several factors contribute to the differences in reactivity among metals:
-
Atomic Radius: As you move down a group in the periodic table, the atomic radius increases. This means the outermost electrons are further from the nucleus and experience a weaker attraction, making them easier to lose. This explains the increase in reactivity down the alkali and alkaline earth metal groups.
-
Ionization Energy: This is the energy required to remove an electron from an atom. Lower ionization energy means it's easier to remove an electron, leading to higher reactivity. Alkali metals have very low ionization energies.
-
Electronegativity: This is the ability of an atom to attract electrons. Metals have low electronegativity, meaning they are less likely to attract electrons and more likely to lose them.
-
Shielding Effect: Inner electrons shield the outermost electrons from the positive charge of the nucleus, reducing the attraction and making the outermost electrons easier to lose.
Conclusion: A Reactive World
The reactivity of metals is a fundamental concept in chemistry with significant implications in various fields, from industrial processes to biological systems. The alkali metals, particularly francium, stand out as the most reactive, showcasing the dramatic consequences of readily available valence electrons. Understanding these principles helps us predict and control chemical reactions, leading to the development of new materials and technologies. While francium's extreme radioactivity limits practical experimentation, the reactivity trend within the alkali and alkaline earth metals offers a clear demonstration of periodic table principles and the fascinating interplay of electronic structure and chemical behavior. The study of reactive metals continues to be a fertile ground for scientific discovery and technological innovation.
Latest Posts
Latest Posts
-
Match Each Atom Or Molecule With Its Correct Description
Apr 01, 2025
-
An Electrical Motor Provides 0 50 W Of Mechanical Power Quizlet
Apr 01, 2025
-
Percent Water In A Hydrate Lab Answer Key
Apr 01, 2025
-
Formula De La Fuerza De Gravedad
Apr 01, 2025
-
Describe How Water Moves Up A Tree Through The Xylem
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Are The Most Reactive Metals On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
