What Are The P Block Elements
Muz Play
Mar 25, 2025 · 6 min read
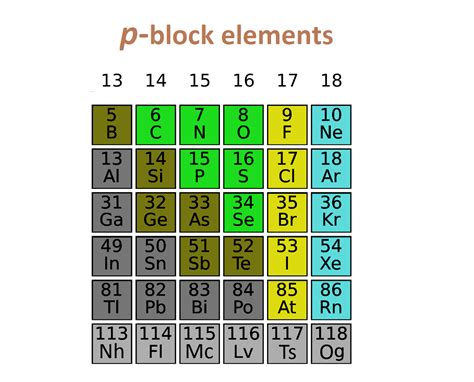
Table of Contents
What are the p-block elements? A Deep Dive into Group 13-18
The periodic table is a cornerstone of chemistry, organizing elements based on their atomic structure and properties. Within this grand organization, the p-block elements hold a special place, exhibiting a fascinating array of characteristics and applications crucial to our daily lives. This comprehensive guide will delve into the world of p-block elements, exploring their properties, trends, and significance.
Defining the p-block Elements
The p-block elements are found on the right-hand side of the periodic table, encompassing groups 13 to 18. They are defined by the filling of their outermost p-orbitals with electrons. This orbital filling dictates their chemical behavior and the unique properties that distinguish them. Unlike the s-block elements (alkali and alkaline earth metals) which tend to be highly reactive, the p-block elements show a wider range of reactivity, from highly reactive non-metals to relatively inert noble gases.
The number of valence electrons (electrons in the outermost shell) in p-block elements varies from three (Group 13) to eight (Group 18). This variation in valence electrons contributes significantly to the diverse chemical behaviors observed within this block.
Properties of p-block Elements
The properties of p-block elements are diverse and don't follow a uniform trend as neatly as in some other blocks of the periodic table. However, some general observations can be made:
1. Electronegativity:
Electronegativity, the ability of an atom to attract electrons towards itself in a chemical bond, generally increases across a period (from left to right) and decreases down a group (from top to bottom). This means that elements on the far right of the p-block (halogens) are highly electronegative, while those on the far left (Group 13) are relatively less electronegative. This trend is important in determining the type of bonding (ionic, covalent) that these elements will form.
2. Ionization Energy:
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period and decreases down a group, similar to electronegativity. This reflects the increasing nuclear charge and the resulting stronger attraction to electrons across a period.
3. Metallic Character:
Metallic character generally decreases from left to right across a period and increases down a group. Group 13 elements show some metallic properties, while the elements towards the right become increasingly non-metallic in nature. The transition from metallic to non-metallic behavior is gradual, leading to the existence of metalloids (semi-metals) like silicon and germanium.
4. Oxidation States:
The oxidation states of p-block elements are diverse and depend on the number of valence electrons available for bonding. Elements can exhibit multiple oxidation states, often leading to a variety of compounds with different properties. For instance, phosphorus can exist in oxidation states ranging from -3 to +5.
5. Reactivity:
The reactivity of p-block elements varies greatly depending on their position in the block. Group 14 elements (carbon, silicon, germanium, etc.) exhibit relatively low reactivity compared to other groups. Halogens (Group 17) are highly reactive non-metals, readily forming compounds with most other elements. Noble gases (Group 18) are exceptionally unreactive due to their full valence shells.
Individual Group Analysis: A Deeper Dive
Let's examine each group within the p-block in more detail:
Group 13 (Boron Group):
This group includes boron, aluminum, gallium, indium, and thallium. Boron is a metalloid, while the others are metals. They are relatively less reactive than other p-block elements and often exhibit +3 oxidation state. Aluminum is particularly important in everyday applications, such as in cans and cookware.
Group 14 (Carbon Group):
This group contains carbon, silicon, germanium, tin, and lead. Carbon is unique due to its ability to form long chains and complex structures, leading to the vast field of organic chemistry. Silicon is a crucial component in semiconductors and computer chips. Tin and lead are traditional metals used in alloys and other applications.
Group 15 (Nitrogen Group):
Nitrogen, phosphorus, arsenic, antimony, and bismuth comprise this group. Nitrogen is a vital element in biological systems, forming a key component of amino acids and proteins. Phosphorus is crucial in DNA and RNA, and also in fertilizers. Arsenic, antimony, and bismuth exhibit a wider range of oxidation states and are less common in everyday applications.
Group 16 (Chalcogens):
Oxygen, sulfur, selenium, tellurium, and polonium belong to this group. Oxygen is essential for life, playing a vital role in respiration. Sulfur is found in various compounds, including sulfuric acid, a crucial industrial chemical. Selenium and tellurium are used in specific applications, such as semiconductors and photocopiers.
Group 17 (Halogens):
Fluorine, chlorine, bromine, iodine, and astatine form this group, all highly reactive nonmetals. Halogens are potent oxidizing agents and readily form salts with metals. Chlorine is used in water purification, while iodine is essential for thyroid function.
Group 18 (Noble Gases):
Helium, neon, argon, krypton, xenon, and radon are extremely unreactive due to their full valence electron shells. They are used in various applications, such as lighting (neon) and cryogenics (helium). Their inertness makes them useful in protecting reactive substances.
Applications of p-block Elements
The applications of p-block elements are vast and integral to modern society. Here are some notable examples:
- Electronics: Silicon and germanium are indispensable in the semiconductor industry, forming the basis of transistors and integrated circuits. Other p-block elements are also used in specialized electronic components.
- Medicine: Several p-block elements and their compounds have medicinal applications. For example, iodine is essential for thyroid hormone production, while certain phosphorus compounds are used in pharmaceuticals.
- Agriculture: Phosphorus and nitrogen are essential nutrients for plant growth, with phosphorus compounds being crucial components of fertilizers.
- Industry: Many industrial processes rely on p-block elements. Sulfuric acid production, for instance, is a cornerstone of the chemical industry. Aluminum is widely used in construction and packaging.
- Everyday life: Many common materials contain p-block elements. For instance, glass contains silicon, and many plastics incorporate carbon.
Trends and Anomalies within the p-block
While general trends are observed within the p-block, exceptions and anomalies exist, highlighting the complexities of chemical behavior. For instance, the relatively high ionization energy of nitrogen compared to oxygen is attributed to its half-filled p-orbital configuration, which is relatively stable. Similar anomalies exist for other elements within the p-block, showcasing the intricate interplay of electronic configurations and interatomic forces.
Conclusion: The Importance of p-block Elements
The p-block elements represent a diverse and crucial group of elements underpinning many aspects of our lives. Their varied properties, ranging from the extreme reactivity of halogens to the inertness of noble gases, enable a wide range of applications, impacting diverse fields from electronics and medicine to agriculture and industry. Understanding the characteristics and trends within the p-block is essential for advancements in various scientific and technological domains. The continued study and exploration of these elements will undoubtedly lead to further discoveries and innovations in the future. Further research into the intricacies of their chemical behaviour promises exciting breakthroughs in material science, medicine, and various other fields. This deep dive has only scratched the surface of the fascinating world of p-block elements; further investigation will reveal even greater depths to their complexity and importance.
Latest Posts
Latest Posts
-
Are Double Bonds Stronger Than Single Bonds
Mar 27, 2025
-
Consumer Surplus With A Price Floor
Mar 27, 2025
-
Ground State Electron Configuration Of C
Mar 27, 2025
-
Solving Linear Systems With Graphing 7 1
Mar 27, 2025
-
Compare And Contrast Sexual Reproduction And Asexual Reproduction
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Are The P Block Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
