What Are The Vertical Columns Called On A Periodic Table
Muz Play
Mar 29, 2025 · 7 min read
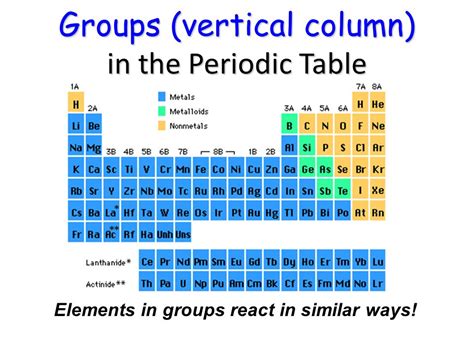
Table of Contents
What Are the Vertical Columns Called on a Periodic Table?
The periodic table, a cornerstone of chemistry, organizes chemical elements in a structured format that reveals patterns in their properties. Understanding its organization is crucial for grasping the fundamental principles of chemistry. One of the key aspects of this organization lies in the vertical columns, which are known as groups or families. This article will delve into the details of these vertical columns, exploring their naming conventions, characteristic properties, and the underlying reasons for their similar behaviors.
Understanding Groups and Families: The Vertical Organization
The vertical columns, or groups, on the periodic table represent elements with similar chemical properties. These similarities stem from the fact that elements within the same group have the same number of valence electrons – electrons in their outermost shell. These valence electrons are the key players in chemical bonding, determining how an atom will interact with other atoms to form molecules and compounds. Because of this shared characteristic, elements in the same group tend to exhibit similar reactivity and form similar types of compounds.
Another term often used interchangeably with "group" is "family". This terminology highlights the close relationship and shared characteristics among the elements within a column. While both terms are perfectly acceptable, "group" is generally preferred in formal scientific contexts.
The 18 Groups of the Periodic Table: A Detailed Look
The modern periodic table consists of 18 numbered groups, each with its unique set of properties. Let's explore some of the key groups and their defining characteristics:
Group 1: The Alkali Metals
This group houses the most reactive metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Their reactivity stems from having only one valence electron, which they readily lose to form +1 ions. This makes them highly reactive with water and air, often exhibiting vigorous reactions. They are soft, silvery-white metals with low melting points.
Group 2: The Alkaline Earth Metals
Slightly less reactive than the alkali metals, the alkaline earth metals – beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) – each have two valence electrons. They tend to lose these two electrons to form +2 ions. While still reactive, their reactions are generally less vigorous than those of the alkali metals. They are also harder and have higher melting points than the alkali metals.
Group 17: The Halogens
The halogens – fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) – are highly reactive nonmetals. They have seven valence electrons and tend to gain one electron to form -1 ions, achieving a stable electron configuration. Their reactivity decreases down the group, with fluorine being the most reactive. They exist in various states of matter at room temperature: fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid.
Group 18: The Noble Gases
The noble gases – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) – are unique for their exceptional stability. They have a full outer electron shell (eight valence electrons, except for helium, which has two), making them extremely unreactive. This inertness is why they are often called "inert gases". They are all gases at room temperature and are generally colorless and odorless.
Transition Metals (Groups 3-12)
The transition metals occupy the central block of the periodic table. They are characterized by their variable oxidation states, meaning they can lose different numbers of electrons to form ions with various charges. This versatility allows them to form a wide range of compounds with diverse properties. Many transition metals are known for their colorful compounds and catalytic properties. Examples include iron (Fe), copper (Cu), gold (Au), and platinum (Pt).
Inner Transition Metals (Lanthanides and Actinides)
These elements are placed separately at the bottom of the periodic table to avoid excessive horizontal stretching. The lanthanides are known for their similar chemical properties and are often difficult to separate. The actinides are all radioactive and many are synthetically produced.
Other Important Groups
Other groups also exhibit distinct properties and play crucial roles in various applications. Group 14 (carbon group) contains carbon, the basis of organic chemistry; Group 15 (nitrogen group) includes nitrogen, a vital component of the atmosphere; and Group 16 (chalcogens) includes oxygen, essential for respiration.
The Significance of Group Numbering
The numbering system for groups reflects the number of valence electrons for the representative elements (main group elements). For example, Group 1 elements have one valence electron, Group 2 elements have two, and so on. This pattern breaks down slightly for the transition metals, where the number of valence electrons is less straightforward. However, the numbering system provides a convenient way to categorize and compare elements based on their electron configuration.
Trends within Groups: Periodic Properties
As you move down a group in the periodic table, certain periodic properties exhibit predictable trends:
- Atomic Radius: Atomic radius generally increases down a group. This is because additional electron shells are added, increasing the distance between the nucleus and the outermost electrons.
- Ionization Energy: Ionization energy, the energy required to remove an electron, generally decreases down a group. The increased atomic radius means the outermost electrons are further from the nucleus and less tightly held.
- Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally decreases down a group. The increased atomic radius means the nucleus has less influence on the bonding electrons.
- Metallic Character: Metallic character generally increases down a group. Elements become more metallic as you move down, exhibiting properties like conductivity and malleability.
Importance of Group Classification in Chemistry
Understanding the groups of the periodic table is vital for several reasons:
- Predicting Chemical Behavior: Knowing the group of an element allows chemists to predict its reactivity and the types of compounds it will form.
- Designing Materials: The properties of elements within a group can be exploited in materials science to design materials with specific characteristics.
- Understanding Chemical Reactions: Groups provide a framework for understanding chemical reactions and predicting the products of those reactions.
- Technological Applications: The properties of elements in specific groups are used in various technological applications, such as in electronics, medicine, and energy production.
Beyond the Basics: A Deeper Dive into Group Properties
The characteristics outlined above provide a foundational understanding of group properties. However, a deeper investigation reveals more nuanced behaviors and exceptions to the general trends. For instance:
- Anomalous Behavior of First Members: The first element in each group often displays different properties compared to the rest of the group. This is attributed to its smaller size and higher electronegativity. For example, Lithium's behavior differs significantly from other alkali metals.
- Influence of d and f Orbitals: The properties of transition metals and inner transition metals are significantly influenced by the involvement of d and f orbitals in bonding. This leads to a greater variety of oxidation states and complex chemical behaviors.
- Relativistic Effects: In heavier elements, relativistic effects, stemming from the high speed of inner electrons, can affect their properties and reactivity. These effects are particularly pronounced for the heavier elements at the bottom of certain groups.
Conclusion: Mastering the Vertical Columns for Chemical Understanding
The vertical columns, or groups, on the periodic table are not simply a convenient arrangement of elements. They are a fundamental organizational principle reflecting the underlying relationships between elements based on their electron configurations and shared properties. Understanding these groups, their characteristic trends, and the nuances of their behaviors is essential for anyone seeking a deeper grasp of chemical principles. By mastering the organization and properties of these vertical columns, you unlock a key to understanding the vast world of chemistry and its countless applications. Further exploration into individual groups and their unique characteristics will deepen your understanding and unlock a wealth of knowledge about the building blocks of our world.
Latest Posts
Latest Posts
-
Lab Skills Using A Graduated Cylinder
Apr 01, 2025
-
Which Molecules In Eukaryotic Cells Regulate Gene Expression
Apr 01, 2025
-
How Do Natural Killer Cells Destroy Invading Pathogens
Apr 01, 2025
-
What Stimulates The Pollen Tube To Grow
Apr 01, 2025
-
How To Find C In Sinusoidal Function
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Are The Vertical Columns Called On A Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
