What Classification Of Alcohol Is Resistant To Oxidation
Muz Play
Mar 20, 2025 · 5 min read
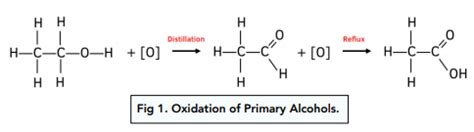
Table of Contents
What Classification of Alcohol is Resistant to Oxidation?
Oxidation is a chemical process that involves the loss of electrons from a molecule. In the context of alcohols, oxidation typically involves the conversion of the hydroxyl group (-OH) to a carbonyl group (C=O), forming aldehydes or ketones. The susceptibility of an alcohol to oxidation depends heavily on its structure, specifically the type of carbon atom the hydroxyl group is attached to. Understanding this relationship is crucial in various fields, including organic chemistry, biochemistry, and even the production and preservation of alcoholic beverages. This article delves into the classification of alcohols and their varying resistance to oxidation.
Understanding Alcohol Classification
Alcohols are classified based on the number of alkyl groups attached to the carbon atom bearing the hydroxyl group (-OH). This classification directly impacts their oxidation behavior.
1. Primary Alcohols (1°)
Primary alcohols have the hydroxyl group attached to a primary carbon atom—a carbon atom bonded to only one other carbon atom. This makes them the most susceptible to oxidation. Oxidation of a primary alcohol typically proceeds in two steps:
- Step 1: Oxidation to an aldehyde. This step is relatively easy and often proceeds quickly.
- Step 2: Further oxidation of the aldehyde to a carboxylic acid. This step is also relatively easy, especially in the presence of strong oxidizing agents.
Example: Ethanol (CH₃CH₂OH), the alcohol found in alcoholic beverages, is a primary alcohol. It readily oxidizes to acetaldehyde (CH₃CHO) and then to acetic acid (CH₃COOH).
2. Secondary Alcohols (2°)
Secondary alcohols have the hydroxyl group attached to a secondary carbon atom—a carbon atom bonded to two other carbon atoms. They are less susceptible to oxidation than primary alcohols. Oxidation of a secondary alcohol produces a ketone. This reaction generally stops at the ketone stage; further oxidation is difficult.
Example: Isopropanol (CH₃CHOHCH₃), commonly used as rubbing alcohol, is a secondary alcohol. Oxidation yields acetone (CH₃COCH₃).
3. Tertiary Alcohols (3°)
Tertiary alcohols have the hydroxyl group attached to a tertiary carbon atom—a carbon atom bonded to three other carbon atoms. They are resistant to oxidation under typical conditions. The reason for this resistance lies in the lack of a hydrogen atom on the carbon bearing the hydroxyl group. The oxidation process requires the removal of a hydrogen atom from the carbon atom adjacent to the hydroxyl group, a step that is impossible in tertiary alcohols. Therefore, they do not readily undergo oxidation reactions with common oxidizing agents like potassium dichromate (K₂Cr₂O₇) or potassium permanganate (KMnO₄).
Example: Tert-butanol ((CH₃)₃COH) is a tertiary alcohol and is highly resistant to oxidation.
Factors Influencing Oxidation Resistance
While the classification of alcohol provides a primary indicator of oxidation resistance, other factors also play a role:
- Strength of the Oxidizing Agent: Stronger oxidizing agents can overcome the inherent resistance of some alcohols. For example, while tertiary alcohols are generally resistant to mild oxidizing agents, they can be oxidized under more forcing conditions using strong oxidizing agents such as chromium trioxide (CrO₃) in the presence of strong acids. However, these reactions often lead to complex mixtures and are less predictable.
- Reaction Conditions: Temperature, pH, and the presence of catalysts can significantly influence the rate and extent of oxidation. Higher temperatures generally accelerate the reaction.
- Steric Hindrance: Bulky groups around the hydroxyl group can hinder the approach of the oxidizing agent, reducing the rate of oxidation. This is especially relevant in secondary and tertiary alcohols.
- Solvent Effects: The solvent used in the reaction can affect the reactivity of both the alcohol and the oxidizing agent. Polar solvents generally favor oxidation reactions.
Applications and Implications
The varying oxidation resistance of alcohols has numerous implications across diverse fields:
1. Organic Synthesis
Understanding the oxidation behavior of alcohols is crucial in organic synthesis. Chemists strategically utilize different oxidizing agents and reaction conditions to selectively oxidize specific alcohols while leaving others untouched. This allows for the controlled synthesis of various carbonyl compounds.
2. Biochemistry
In biological systems, the oxidation of alcohols plays a vital role in metabolic pathways. Enzymes specifically catalyze the oxidation of alcohols, often with high selectivity. For example, alcohol dehydrogenase oxidizes ethanol to acetaldehyde, a crucial step in alcohol metabolism.
3. Food and Beverage Industry
The oxidation of alcohols is a critical factor in the production and preservation of alcoholic beverages. Oxidation can lead to undesirable changes in flavor, aroma, and color. The resistance of certain alcohols to oxidation is exploited in the production of stable beverages. For instance, the resistance of tertiary alcohols to oxidation contributes to the stability of some flavouring compounds.
4. Pharmaceutical Industry
Many pharmaceuticals contain alcohol functional groups. Understanding the oxidation stability of these molecules is essential for ensuring the shelf life and efficacy of the drug product. Oxidation can lead to the degradation of the active pharmaceutical ingredient, reducing its potency or creating harmful byproducts.
Conclusion
The classification of alcohols—primary, secondary, and tertiary—provides a valuable framework for predicting their resistance to oxidation. Tertiary alcohols exhibit the highest resistance due to the absence of a hydrogen atom on the carbon bearing the hydroxyl group. However, the strength of the oxidizing agent, reaction conditions, steric hindrance, and solvent effects also significantly influence oxidation behavior. Understanding these factors is critical in various applications, including organic synthesis, biochemistry, food science, and pharmaceuticals, allowing for better control and prediction of alcohol oxidation reactions. The selective oxidation of alcohols remains a powerful tool in chemical synthesis and a vital process in numerous biological and industrial settings. Further research continues to refine our understanding of alcohol oxidation, pushing the boundaries of organic chemistry and its applications.
Latest Posts
Latest Posts
-
How To Do Data Transformations Statistics In Excel
Mar 21, 2025
-
Find All Real Zeros Of The Function
Mar 21, 2025
-
Does Electronegativity Increase Down A Group
Mar 21, 2025
-
Limiting Reactant And Percent Yield Practice
Mar 21, 2025
-
The Long Love That In My Thought Doth Harbor
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Classification Of Alcohol Is Resistant To Oxidation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
