What Determines The Function Of A Protein
Muz Play
Mar 25, 2025 · 6 min read
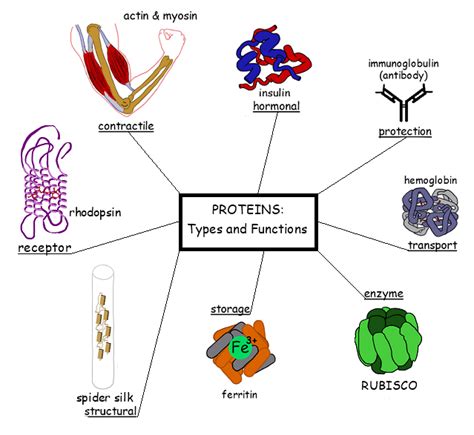
Table of Contents
What Determines the Function of a Protein?
Proteins are the workhorses of the cell, carrying out a vast array of functions essential for life. From catalyzing biochemical reactions to providing structural support, their diverse roles stem from their intricate three-dimensional structures and specific chemical properties. But what exactly determines the function of a protein? The answer is multifaceted and involves a complex interplay of factors, starting from the very sequence of amino acids that make up the protein.
The Primary Structure: The Foundation of Protein Function
The fundamental determinant of a protein's function is its primary structure: the linear sequence of amino acids. This sequence is dictated by the genetic code encoded in DNA. Each amino acid possesses unique chemical properties: some are hydrophobic (water-repelling), others hydrophilic (water-attracting), some are positively charged, others negatively charged, and some have bulky side chains while others are small. This diversity in amino acid properties is crucial.
The Impact of Amino Acid Sequence
The precise order of these amino acids dictates how the protein will fold and interact with other molecules. Even a single amino acid substitution can dramatically alter the protein's function, as seen in sickle cell anemia, where a single glutamic acid is replaced by valine in the beta-globin protein, causing the red blood cells to become sickle-shaped and impairing their oxygen-carrying capacity.
Key takeaway: The primary structure acts as a blueprint, determining all subsequent levels of protein structure and ultimately, the protein's function. Variations in this sequence lead to variations in function, highlighting the critical role of genetic information in protein synthesis and function.
Higher-Order Structures: Shaping Protein Function
The linear amino acid chain doesn't simply exist as a straight line. Instead, it folds into intricate three-dimensional structures, driven by interactions between the amino acid side chains. These higher-order structures are essential for protein function.
Secondary Structure: Local Folding Patterns
The primary structure begins to fold into local patterns called secondary structures. These are stabilized by hydrogen bonds between the backbone atoms of the amino acid chain. The most common secondary structures are:
- Alpha-helices: Coiled structures resembling a spring, stabilized by hydrogen bonds between every fourth amino acid.
- Beta-sheets: Flat, pleated structures formed by hydrogen bonds between adjacent polypeptide chains or segments of the same chain.
- Random coils: Regions of the protein that lack a defined secondary structure, often crucial for flexibility and interactions with other molecules.
The arrangement and proportion of these secondary structures significantly contribute to the overall shape and function of the protein.
Tertiary Structure: The 3D Arrangement
The next level of organization is the tertiary structure, which represents the overall three-dimensional arrangement of the polypeptide chain. This structure is determined by a complex interplay of various forces, including:
- Hydrophobic interactions: Hydrophobic amino acids tend to cluster together in the protein's core, away from the surrounding water molecules.
- Hydrogen bonds: These form between various polar groups in the amino acid side chains.
- Ionic bonds (salt bridges): These occur between oppositely charged amino acid side chains.
- Disulfide bonds: Covalent bonds formed between cysteine residues, providing significant stability to the protein structure.
The tertiary structure is critical for creating functional domains within the protein. These domains are distinct structural and functional units that often perform specific tasks, such as binding to a ligand or catalyzing a reaction.
Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these chains constitutes the quaternary structure. Examples include hemoglobin, with its four subunits, and many enzymes with multiple subunits working cooperatively.
The interactions between subunits in the quaternary structure are similar to those in the tertiary structure, involving hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. The quaternary structure is often crucial for regulating protein activity and stability.
Post-Translational Modifications: Fine-tuning Protein Function
Even after the protein has achieved its correct three-dimensional structure, its function can be further modulated by post-translational modifications (PTMs). These are chemical modifications that occur after the protein has been synthesized. Common PTMs include:
- Phosphorylation: The addition of a phosphate group, often altering the protein's activity or localization.
- Glycosylation: The addition of sugar molecules, affecting protein stability, solubility, and interactions with other molecules.
- Ubiquitination: The attachment of ubiquitin, a small protein that marks the target protein for degradation.
- Acetylation: The addition of an acetyl group, which can alter protein stability and function.
These modifications can act as molecular switches, activating or deactivating the protein, altering its location within the cell, or targeting it for degradation. They provide a level of fine-tuning and dynamic regulation of protein function that is essential for cellular processes.
Environmental Factors: Influences on Protein Conformation and Function
The environment in which a protein exists significantly influences its structure and function. Several key factors play a role:
pH:
Changes in pH can alter the charge of amino acid side chains, affecting electrostatic interactions and potentially disrupting the protein's structure. This can lead to denaturation, where the protein loses its three-dimensional structure and its function.
Temperature:
High temperatures can disrupt the weak interactions holding the protein together, leading to denaturation. Conversely, extremely low temperatures can slow down protein function. Each protein has an optimal temperature range for its function.
Ionic Strength:
The concentration of ions in the surrounding solution can affect electrostatic interactions within the protein, impacting its stability and function.
Presence of Ligands:
The binding of ligands (small molecules or other proteins) to specific sites on the protein can induce conformational changes, altering the protein's activity. This is a crucial mechanism for regulating protein function.
Chaperones:
Cellular chaperone proteins assist in the correct folding of newly synthesized proteins, preventing aggregation and misfolding. Their function is crucial for maintaining the integrity and functionality of the proteome.
Protein-Protein Interactions: Orchestrating Cellular Processes
Many proteins don't function in isolation; instead, they interact with other proteins to perform their roles. These protein-protein interactions are crucial for a wide range of cellular processes, from signal transduction to DNA replication.
The specificity of these interactions is determined by the shape and chemical properties of the interacting surfaces on the proteins. The strength of the interaction can range from transient and weak to strong and stable, depending on the specific proteins and their function. These interactions are often mediated by various types of bonds, similar to those involved in tertiary and quaternary structures.
Conclusion: A Holistic View of Protein Function
In summary, the function of a protein is a complex outcome determined by a combination of factors, all intricately interconnected. From the primary amino acid sequence, which dictates higher-order structures, to post-translational modifications and the influence of environmental factors, understanding each component is crucial to appreciating the remarkable diversity and complexity of protein function. Protein-protein interactions further add to this complexity, highlighting the highly orchestrated nature of cellular processes. Continued research in this field continues to unravel the intricate mechanisms governing protein structure, function, and regulation, providing valuable insights into various biological processes and potential therapeutic targets.
Latest Posts
Latest Posts
-
How To Do Bohr Rutherford Diagrams
May 12, 2025
-
Is Milk Pure Substance Or Mixture
May 12, 2025
-
Power Series Of 1 1 X
May 12, 2025
-
Is Boron Trifluoride Polar Or Nonpolar
May 12, 2025
-
Which Point Of The Beam Experiences The Most Compression
May 12, 2025
Related Post
Thank you for visiting our website which covers about What Determines The Function Of A Protein . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.