What Factors Influence The Rate Of Diffusion
Muz Play
Mar 23, 2025 · 6 min read
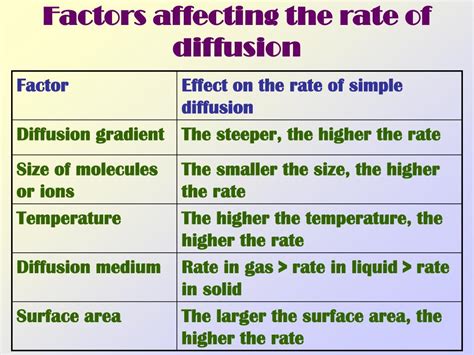
Table of Contents
What Factors Influence the Rate of Diffusion?
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in many natural systems. Understanding the factors influencing its rate is crucial in various fields, from biology and chemistry to engineering and environmental science. This comprehensive guide will delve into the key parameters that govern the speed and efficiency of diffusion.
Key Factors Affecting Diffusion Rate
Several factors intricately interact to determine the rate of diffusion. These can be broadly categorized into:
1. Concentration Gradient: The Driving Force
The concentration gradient, the difference in concentration between two regions, is the primary driving force behind diffusion. A steeper gradient, meaning a larger difference in concentration, results in a faster diffusion rate. Imagine dropping a dye tablet into a glass of water: the dye spreads rapidly initially because the concentration difference is significant. As the dye disperses, the gradient lessens, and the diffusion rate slows down. This relationship is often described mathematically using Fick's First Law of Diffusion.
2. Temperature: Molecular Kinetic Energy
Temperature significantly impacts the rate of diffusion. Higher temperatures mean molecules possess greater kinetic energy, leading to faster movement and more frequent collisions. This increased molecular motion translates to a higher diffusion rate. Conversely, at lower temperatures, molecules move more slowly, resulting in a slower diffusion rate. This is why, for example, sugar dissolves faster in hot water than in cold water. The heightened kinetic energy at higher temperatures overcomes the intermolecular forces holding the sugar molecules together, accelerating the diffusion process.
3. Mass of the Diffusing Substance: Size Matters
The mass of the diffusing particles plays a crucial role. Smaller molecules diffuse faster than larger ones. This is because smaller molecules possess a higher average kinetic energy at a given temperature and experience less resistance as they navigate through the medium. Consider the diffusion of gases: smaller gas molecules like helium diffuse faster than larger gas molecules like xenon under the same conditions. This size dependence is inversely proportional—smaller mass equates to faster diffusion.
4. Distance: The Barrier to Movement
The distance over which diffusion must occur also affects the rate. The greater the distance, the longer it takes for particles to travel from a region of high concentration to a region of low concentration. This is because diffusion is a relatively slow process, especially over large distances. Consider the challenges faced by nutrients diffusing to the innermost cells of a large organism. Efficient transport mechanisms, beyond simple diffusion, are essential in such instances.
5. Medium of Diffusion: Resistance to Movement
The medium through which diffusion takes place significantly impacts the rate. Different media offer varying degrees of resistance to molecular movement. Diffusion occurs faster in gases than in liquids, and faster in liquids than in solids. This is due to the different degrees of freedom and intermolecular forces present in each state of matter. Gases have weak intermolecular forces and large spaces between molecules, facilitating rapid diffusion. Liquids have stronger intermolecular forces, leading to slower diffusion. Solids, with their rigid structure, present the greatest resistance to molecular movement, leading to the slowest diffusion rates.
6. Surface Area: Access to Diffusion
The surface area available for diffusion is another critical factor. A larger surface area provides more avenues for particles to move from one region to another, thus accelerating the process. This principle is exemplified in the design of the lungs, where the extensive network of alveoli provides a vast surface area for efficient gas exchange. Similarly, the folded structure of the small intestine maximizes surface area for nutrient absorption.
7. Pressure: Impacting Molecular Density
In the context of gases, pressure plays a crucial role. Higher pressure increases the density of gas molecules, leading to a higher collision frequency and faster diffusion. This is because, at higher pressures, there are more molecules packed into a given volume, making them more likely to interact and move across concentration gradients. This effect is observed in various industrial processes where pressure is manipulated to control the rate of gaseous diffusion.
8. Permeability of the Membrane (for Membrane Diffusion)
When diffusion occurs across a membrane, the permeability of the membrane becomes paramount. The membrane's structure and composition determine which molecules can pass through and at what rate. Some membranes are highly permeable to certain substances, while others are impermeable. The presence of specific channels or carriers within the membrane can further enhance the permeability of certain molecules. This is particularly important in biological systems, where selective permeability of cell membranes regulates the entry and exit of substances.
9. Presence of Other Solutes: Competitive Effects
The presence of other solutes in the diffusion medium can influence the diffusion rate. This is primarily due to competitive interactions among different molecules for the available space and the potential for molecular interactions to affect movement. For instance, the presence of other dissolved ions in a solution can influence the diffusion rate of a specific ion. These interactions can be complex and may lead to either acceleration or deceleration of diffusion depending on the specific molecules involved.
Applications and Real-World Examples
Understanding the factors influencing diffusion rate has profound implications in numerous fields:
-
Medicine: Drug delivery systems rely on diffusion for efficient drug absorption. Factors like particle size, concentration gradients, and membrane permeability are carefully considered to optimize drug efficacy.
-
Environmental Science: The diffusion of pollutants in air and water bodies governs their distribution and impact on ecosystems. Factors like wind patterns, water currents, and the nature of the pollutant itself influence the spread of contamination.
-
Food Science: The preservation and processing of food involves understanding diffusion processes, such as the diffusion of salt into meat during curing or the diffusion of aroma compounds during the ripening of fruit.
-
Material Science: The synthesis and properties of many materials are influenced by diffusion processes, such as the diffusion of dopants into semiconductors to alter their electrical properties.
-
Biology: Diffusion is fundamental to numerous biological processes, including gas exchange in the lungs, nutrient absorption in the gut, and the transmission of nerve impulses. Understanding diffusion is essential in comprehending cellular function and organismal physiology.
Conclusion
The rate of diffusion is a complex phenomenon determined by the interplay of several interacting factors. A thorough understanding of these factors—concentration gradient, temperature, mass of diffusing substance, distance, medium of diffusion, surface area, pressure, membrane permeability, and presence of other solutes—is crucial for comprehending and manipulating diffusion processes across a wide range of scientific and engineering disciplines. By carefully controlling these parameters, it’s possible to optimize diffusion for diverse applications, from improving drug delivery to minimizing environmental pollution. Further research into the intricacies of diffusion continues to unveil new insights and pave the way for innovative technological advancements.
Latest Posts
Latest Posts
-
Coordination Number In Face Centered Cubic
Mar 25, 2025
-
This Is The Functional Unit Of The Kidney
Mar 25, 2025
-
The Light Dependent Reactions Occur In The Stroma Of The Chloroplast
Mar 25, 2025
-
Name Two Ecological Roles Of Fungi
Mar 25, 2025
-
What Affects The Rate Of Diffusion
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Factors Influence The Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
