What Happens To The Temperature During A Phase Change
Muz Play
Mar 30, 2025 · 7 min read
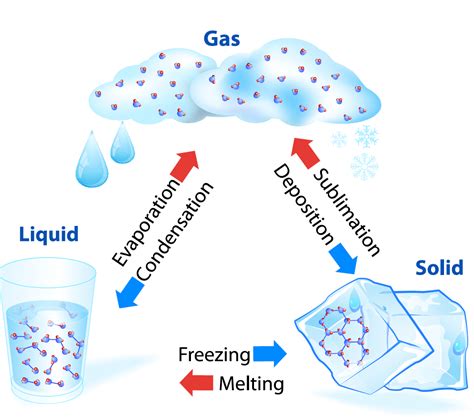
Table of Contents
What Happens to Temperature During a Phase Change?
Understanding phase changes is crucial in various scientific fields, from meteorology to materials science. This comprehensive article delves deep into the fascinating phenomenon of phase transitions, specifically focusing on the seemingly paradoxical behavior of temperature during these transformations. We will explore the underlying principles, using real-world examples to illustrate the concepts.
The Fundamentals of Phase Changes
A phase change, or phase transition, is a physical process in which a substance transforms from one state of matter to another. The three fundamental states are solid, liquid, and gas (we'll also briefly touch upon plasma). These changes are driven by the addition or removal of energy, typically in the form of heat. The key aspect to remember is that during a phase change, the temperature remains constant despite the continued addition or removal of heat. This seemingly counter-intuitive observation is the central theme of this article.
The Role of Latent Heat
The constant temperature during a phase change is explained by the concept of latent heat. Latent heat is the energy absorbed or released during a phase transition without any change in temperature. This energy is used to overcome the intermolecular forces holding the substance together in its current phase. For example, when ice melts, the latent heat of fusion is absorbed to break the strong hydrogen bonds holding the water molecules in their rigid crystalline structure. This energy doesn't increase the kinetic energy of the molecules (and thus doesn't raise the temperature), but rather weakens the bonds, allowing for a change in state.
Types of Latent Heat:
- Latent Heat of Fusion: The energy absorbed during melting (solid to liquid) or released during freezing (liquid to solid).
- Latent Heat of Vaporization: The energy absorbed during vaporization (liquid to gas) or released during condensation (gas to liquid).
- Latent Heat of Sublimation: The energy absorbed during sublimation (solid to gas) or released during deposition (gas to solid).
These latent heats are substance-specific and depend on factors like the strength of intermolecular forces and the molecular structure. Water, for instance, has a relatively high latent heat of vaporization, explaining why sweating is an effective cooling mechanism.
A Deeper Dive into the Process: Molecular Perspective
To fully grasp what happens at the molecular level during a phase change, consider the kinetic theory of matter. This theory states that matter is composed of tiny particles (atoms or molecules) in constant motion. The kinetic energy of these particles is directly related to temperature: higher kinetic energy means higher temperature.
During a phase change, the energy added or removed doesn't directly increase the kinetic energy of the molecules, which would manifest as a temperature increase. Instead, the energy is used to alter the potential energy of the molecules. This potential energy is associated with the forces of attraction between molecules.
Solids, Liquids, and Gases: A Molecular Comparison
- Solids: In solids, molecules are tightly packed and held together by strong intermolecular forces. They vibrate in fixed positions, with limited movement.
- Liquids: In liquids, molecules are less tightly packed than in solids, and the intermolecular forces are weaker. Molecules can move past each other, allowing for fluidity.
- Gases: In gases, molecules are widely dispersed, with very weak intermolecular forces. They move freely and independently, with high kinetic energy.
The transition from one phase to another involves a significant change in the arrangement and interaction of molecules, requiring a substantial energy input or release to overcome or establish the prevailing intermolecular forces.
Temperature-Time Graphs: Visualizing Phase Changes
A temperature-time graph provides a clear visual representation of what happens to the temperature during a phase change. When a substance is heated at a constant rate, the graph shows a linear increase in temperature until a phase change begins. At this point, the temperature plateaus, remaining constant while the phase transition occurs. Once the phase change is complete, the temperature begins to rise again linearly.
This plateau region represents the absorption of latent heat, where the energy is used to overcome intermolecular forces rather than increasing the kinetic energy. The length of the plateau is directly proportional to the amount of latent heat required for the phase transition. A substance with a high latent heat will exhibit a longer plateau than a substance with a low latent heat.
Real-World Examples Illustrated on Temperature-Time Graphs:
Example 1: Melting Ice
Imagine heating a block of ice. Initially, the temperature of the ice increases linearly until it reaches 0°C (32°F). At this point, the temperature plateaus as the ice melts. The energy added is used to break the hydrogen bonds holding the water molecules in the ice lattice, changing the phase from solid to liquid. Once all the ice has melted, the temperature of the water starts to rise again linearly.
Example 2: Boiling Water
Similarly, when heating water, the temperature increases linearly until it reaches 100°C (212°F) at atmospheric pressure. At this point, the temperature remains constant as the water boils, and the latent heat of vaporization is used to overcome the intermolecular forces holding the water molecules together in the liquid phase. Once all the water has vaporized, the temperature of the steam will start to rise.
Example 3: Sublimation of Dry Ice
Dry ice (solid carbon dioxide) undergoes sublimation, directly transitioning from a solid to a gas without passing through the liquid phase. A temperature-time graph for this process would show a plateau at the sublimation temperature, indicating the absorption of latent heat of sublimation.
Beyond Solids, Liquids, and Gases: Plasma
While less common in everyday life, plasma is the fourth fundamental state of matter. Plasma is an ionized gas, meaning that some of its electrons have been stripped from their atoms, resulting in a mixture of ions and free electrons. Phase transitions involving plasma also exhibit constant temperature regions during the change, although the specifics of the energy transfer and the underlying physics become more complex. For instance, the ionization of a gas requires significant energy input to overcome the attractive forces between electrons and nuclei.
Factors Affecting Phase Transitions
Several factors can influence phase transitions and the associated temperature changes:
- Pressure: Changes in pressure can alter the melting and boiling points of substances. Increased pressure generally increases the boiling point and decreases the melting point.
- Impurities: The presence of impurities in a substance can affect its melting and boiling points. Impurities usually lower the freezing point and raise the boiling point.
- Rate of heating/cooling: While the temperature remains constant during a phase change, the rate at which the heat is added or removed influences the duration of the phase transition. A faster rate will result in a shorter plateau.
Practical Applications and Significance
Understanding phase transitions is critical in numerous applications:
- Meteorology: The phase changes of water (evaporation, condensation, precipitation) are essential to understanding weather patterns.
- Material Science: Phase transitions are crucial in the design and processing of materials, such as the crystallization of polymers or the melting of metals.
- Food Science: Freezing and thawing of food involves phase transitions, impacting food quality and preservation.
- Refrigeration and Air Conditioning: These technologies rely on the absorption and release of latent heat during phase changes of refrigerants.
- Chemistry: Phase diagrams are essential tools for understanding chemical reactions and processes.
Conclusion
The seemingly simple process of a phase change reveals a rich interplay of molecular forces, energy transfer, and thermodynamic principles. The constant temperature during a phase transition, explained by the concept of latent heat, is not a mere anomaly but a fundamental aspect of the behavior of matter. Understanding this behavior is vital in numerous scientific and engineering fields, impacting our daily lives in countless ways. From the weather we experience to the materials we use, phase changes are an integral part of the world around us, and their study continues to yield fascinating insights into the nature of matter.
Latest Posts
Latest Posts
-
Will A Metal Lose Or Gain Electrons
Apr 01, 2025
-
What Do Electric Field Lines Represent
Apr 01, 2025
-
How To Find A Hamilton Circuit
Apr 01, 2025
-
The Positive Subatomic Particle Is The
Apr 01, 2025
-
Which Characteristic Of A Substance Is Considered A Chemical Property
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Happens To The Temperature During A Phase Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
