Will A Metal Lose Or Gain Electrons
Muz Play
Apr 01, 2025 · 6 min read
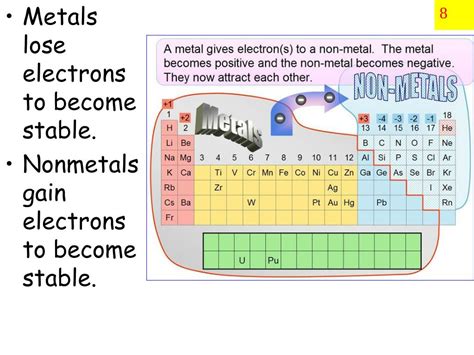
Table of Contents
Will a Metal Lose or Gain Electrons? Understanding Metallic Behavior
The question of whether a metal will lose or gain electrons is fundamental to understanding chemistry and materials science. The short answer is: metals tend to lose electrons, forming positive ions (cations). This behavior is a defining characteristic of metals and dictates their properties and reactivity. However, the full story is far more nuanced and requires a deeper exploration of atomic structure, electron configuration, and the forces governing chemical interactions.
Atomic Structure and Electron Configuration: The Foundation of Metallic Behavior
Atoms are composed of a nucleus containing protons and neutrons, surrounded by electrons orbiting in shells or energy levels. The number of protons determines the element's atomic number and its identity. The electrons, particularly those in the outermost shell (valence electrons), are responsible for chemical bonding and reactivity.
Metals are characterized by having relatively few valence electrons (typically 1-3). These valence electrons are loosely held by the atom's nucleus, making them relatively easy to remove. This is in stark contrast to non-metals, which tend to have many valence electrons and a strong attraction between the nucleus and these electrons.
Electron Configuration and the Octet Rule
The arrangement of electrons in an atom's shells is described by its electron configuration. Atoms strive for stability, often by achieving a full outermost electron shell, a principle known as the octet rule (eight electrons in the valence shell). Metals, with their few valence electrons, find it energetically favorable to lose these electrons, achieving a stable electron configuration resembling that of a noble gas (a group of elements with full outermost electron shells and exceptional stability). This electron loss leads to the formation of positively charged ions (cations).
Ionization Energy: The Energy Required to Remove an Electron
The energy required to remove an electron from a neutral atom is called ionization energy. Metals have relatively low ionization energies compared to non-metals. This low ionization energy reflects the weak attraction between the nucleus and the valence electrons. The lower the ionization energy, the easier it is for the metal to lose an electron. The first ionization energy (removing one electron) is generally lower than subsequent ionization energies (removing additional electrons). This trend reflects the increasing attraction between the positively charged ion and the remaining electrons.
Factors Influencing Electron Loss in Metals
While the general rule is that metals lose electrons, several factors can influence the extent of electron loss and the resulting chemical behavior:
1. Electronegativity: The Tendency to Attract Electrons
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Metals generally have low electronegativity values. When a metal interacts with a non-metal (which has high electronegativity), the non-metal's strong attraction for electrons pulls the electrons away from the metal, leading to electron loss by the metal.
2. Oxidation State: The Charge of an Ion
The oxidation state represents the charge of an ion. Metals can exhibit multiple oxidation states, indicating the ability to lose varying numbers of electrons depending on the specific chemical reaction. For example, iron (Fe) can exist in +2 (ferrous) and +3 (ferric) oxidation states, representing the loss of two or three electrons, respectively.
3. Size and Atomic Radius: Influence on Electron Shielding
The size of an atom, particularly its atomic radius, affects the electron shielding effect. Larger atoms have more electron shells, resulting in greater shielding of the valence electrons from the positive charge of the nucleus. This reduced attraction leads to easier electron loss. As you move down a group in the periodic table, atomic radius increases, and ionization energy decreases, making electron loss more likely.
4. The Nature of the Reactant: The Role of Other Elements
The identity of the reacting element significantly impacts the outcome. Highly electronegative elements, like halogens (fluorine, chlorine, bromine, iodine), readily accept electrons from metals. The interaction between a metal and a highly electronegative non-metal favors the transfer of electrons from the metal to the non-metal.
Examples of Electron Loss in Metals
Many everyday phenomena illustrate the tendency of metals to lose electrons:
-
Rusting of Iron: Iron (Fe) reacts with oxygen (O2) and water (H2O) in the environment, forming iron oxide (rust). During this process, iron atoms lose electrons to oxygen atoms, forming Fe2+ and Fe3+ ions. This is an oxidation-reduction (redox) reaction, where iron is oxidized (loses electrons) and oxygen is reduced (gains electrons).
-
Formation of Metal Oxides: Many metals react with oxygen to form metal oxides. For example, magnesium (Mg) burns brightly in air, reacting with oxygen to form magnesium oxide (MgO). In this reaction, magnesium atoms lose two electrons to oxygen atoms, becoming Mg2+ ions.
-
Reactions with Acids: Metals react with acids, producing hydrogen gas and a metal salt. For example, zinc (Zn) reacts with hydrochloric acid (HCl) to produce zinc chloride (ZnCl2) and hydrogen gas (H2). The zinc atoms lose electrons to hydrogen ions (H+), forming Zn2+ ions.
Exceptional Cases: When Metals Might Appear to Gain Electrons
Although rare, there are exceptional circumstances where metals might seem to gain electrons. This usually doesn't involve a fundamental change in their nature but rather the formation of complex ions or intermetallic compounds:
-
Complex Ion Formation: Some transition metals can form complex ions where they appear to have a negative oxidation state. This is due to the formation of coordinate covalent bonds with ligands (molecules or ions that donate electron pairs). The metal ion acts as an electron acceptor, but the overall process is more complex than simple electron gain.
-
Intermetallic Compounds: These compounds involve combinations of metals, where the electron transfer is not necessarily a complete transfer but rather a sharing or redistribution of electrons in a metallic bond. These situations are better described as electron delocalization rather than straightforward electron gain by a specific metal atom.
Conclusion: The Dominant Trend of Electron Loss in Metals
In the vast majority of chemical reactions, metals exhibit a strong tendency to lose electrons, forming positive ions. This fundamental characteristic is deeply rooted in their atomic structure, low ionization energies, and low electronegativity. The resulting positively charged ions play crucial roles in various chemical and physical processes, influencing the properties of materials and driving numerous chemical reactions. While exceptions exist, particularly involving complex ion formation or intermetallic compounds, the dominant trend for metals remains electron loss. Understanding this fundamental principle is essential for comprehending the behavior of metals and their crucial roles in various scientific and technological applications. This knowledge is crucial in fields ranging from materials science and engineering to biochemistry and environmental science.
Latest Posts
Latest Posts
-
What Is The Conflict Of The Play
Apr 02, 2025
-
The Cell Is The Basic Unit Of
Apr 02, 2025
-
Comparison Of Somatic And Autonomic Nervous Systems Concept Map
Apr 02, 2025
-
How To Convert Molecules To Atoms
Apr 02, 2025
-
Ejemplo De Diagrama De Cuerpo Libre
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Will A Metal Lose Or Gain Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
