What Happens When Acids And Bases Are Mixed
Muz Play
Mar 25, 2025 · 6 min read
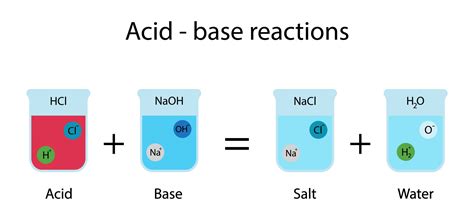
Table of Contents
What Happens When Acids and Bases Mix: A Deep Dive into Neutralization Reactions
Acids and bases are fundamental concepts in chemistry, defining a significant portion of chemical reactions and applications in our daily lives. From the sour taste of lemon juice (citric acid) to the slippery feel of soap (a base), these substances play crucial roles. But what happens when you combine them? The answer is a fascinating chemical reaction known as neutralization. This article delves deep into the process of neutralization, exploring its mechanisms, products, applications, and real-world significance.
Understanding Acids and Bases: A Quick Recap
Before diving into the intricacies of neutralization, let's refresh our understanding of acids and bases. There are several ways to define these substances, but two prominent theories are the Arrhenius and Brønsted-Lowry models.
Arrhenius Definition
The Arrhenius theory, proposed by Svante Arrhenius in 1884, defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, while bases produce hydroxide ions (OH⁻). This definition is simple and effective for many common acids and bases but has limitations when dealing with non-aqueous solutions.
- Examples of Arrhenius acids: Hydrochloric acid (HCl), sulfuric acid (H₂SO₄), nitric acid (HNO₃)
- Examples of Arrhenius bases: Sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)₂)
Brønsted-Lowry Definition
The Brønsted-Lowry theory, developed by Johannes Nicolaus Brønsted and Thomas Martin Lowry independently in 1923, provides a broader definition. It defines acids as proton donors and bases as proton acceptors. This theory expands the scope to include substances that don't necessarily contain hydroxide ions but can still act as bases by accepting protons.
- Example: Ammonia (NH₃) acts as a Brønsted-Lowry base by accepting a proton from water to form ammonium ions (NH₄⁺).
The Magic of Neutralization: What Happens When Acids and Bases Meet?
When an acid and a base are mixed, they undergo a neutralization reaction. This reaction involves the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base to form water (H₂O). The other product is typically a salt, an ionic compound formed from the cation of the base and the anion of the acid.
The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
In this reaction:
- HCl is the acid, donating a proton (H⁺).
- NaOH is the base, accepting a proton (H⁺) through its hydroxide ion (OH⁻).
- NaCl is the salt, sodium chloride (common table salt).
- H₂O is water.
The pH Scale and Neutralization
The pH scale measures the acidity or basicity of a solution. A pH of 7 is considered neutral, while values below 7 indicate acidity and values above 7 indicate basicity. During neutralization, the pH of the solution changes. If a strong acid is neutralized by a strong base, the resulting solution will have a pH of approximately 7. However, if a weak acid or a weak base is involved, the resulting pH might be slightly acidic or basic.
Heat of Neutralization
Neutralization reactions are exothermic, meaning they release heat. This heat release is due to the strong bonds formed during the creation of water molecules. The amount of heat released varies depending on the strength of the acid and base involved. Strong acid-strong base reactions generally release more heat than weak acid-weak base reactions.
Types of Neutralization Reactions and Their Products
Neutralization reactions aren't always straightforward. The nature of the acid and base involved dictates the characteristics of the resulting salt and the overall reaction.
Strong Acid-Strong Base Reactions
These reactions produce neutral salts and water. The pH of the resulting solution is close to 7. Examples include:
- HCl + NaOH → NaCl + H₂O
- HNO₃ + KOH → KNO₃ + H₂O
Weak Acid-Strong Base Reactions
These reactions produce basic salts and water. The pH of the solution will be greater than 7. This is because the conjugate base of the weak acid is still capable of accepting protons, leading to a slightly basic solution. Examples include:
- CH₃COOH + NaOH → CH₃COONa + H₂O (acetic acid and sodium hydroxide)
Strong Acid-Weak Base Reactions
These reactions produce acidic salts and water. The pH of the solution will be less than 7. The conjugate acid of the weak base can donate protons, resulting in a slightly acidic solution. Examples include:
- HCl + NH₃ → NH₄Cl + H₂O (hydrochloric acid and ammonia)
Weak Acid-Weak Base Reactions
These reactions are more complex, and predicting the pH of the resulting solution is less straightforward. It depends on the relative strengths of the acid and the base. The pH can be acidic, basic, or even close to neutral depending on the specific reactants.
Applications of Neutralization Reactions
Neutralization reactions have widespread applications across various fields:
1. Medicine
Antacids, commonly used to relieve heartburn and indigestion, work by neutralizing excess stomach acid (HCl). Ingredients like calcium carbonate (CaCO₃) and magnesium hydroxide (Mg(OH)₂) act as bases, neutralizing the acid and providing relief.
2. Industrial Processes
Neutralization is crucial in many industrial processes:
- Wastewater treatment: Industrial wastewater often contains acidic or basic components. Neutralization helps adjust the pH to environmentally safe levels before discharge.
- Chemical synthesis: Neutralization reactions are frequently used in the synthesis of various chemicals, helping control reaction conditions and isolate products.
- Food industry: pH adjustment through neutralization is vital in food processing for maintaining product quality and safety.
3. Environmental Applications
Acid rain, caused by atmospheric pollutants, can acidify lakes and rivers. Liming, the process of adding calcium carbonate (CaCO₃) to these water bodies, neutralizes the acidity and helps restore the ecosystem.
4. Agriculture
Soil pH significantly impacts plant growth. Farmers often use lime or other neutralizing agents to adjust soil pH to optimal levels for specific crops.
Beyond the Basics: Titration and Equivalence Point
A crucial technique for determining the concentration of an acid or base is titration. In titration, a solution of known concentration (the titrant) is gradually added to a solution of unknown concentration until the reaction is complete, reaching the equivalence point. At the equivalence point, the moles of acid and base are equal, and neutralization is complete. Indicators, such as phenolphthalein, are often used to visually signal the equivalence point.
Safety Considerations
Working with acids and bases requires careful attention to safety. Always wear appropriate protective gear, including gloves and eye protection. Acids and bases can cause burns, so immediate rinsing with plenty of water is essential in case of accidental contact.
Conclusion
The interaction between acids and bases, leading to neutralization reactions, is a fundamental concept in chemistry with far-reaching consequences. Understanding the principles of neutralization is crucial for various applications, from everyday remedies to large-scale industrial processes and environmental remediation. The ability to control and manipulate these reactions allows us to harness their power for beneficial purposes, highlighting the importance of this fundamental chemical process in our world. From the simple act of taking an antacid to complex environmental cleanup efforts, the principles of acid-base neutralization are constantly at work, shaping our lives in subtle yet significant ways. Further exploration into specific acid-base reactions and their applications can unlock even deeper understandings and broaden the range of potential applications in the future.
Latest Posts
Latest Posts
-
How Many Neutrons Does K Have
Mar 27, 2025
-
A Larger Nucleus Splits Apart Making 2 Smaller Ones
Mar 27, 2025
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
-
Song Lyrics Ode To Billy Joe
Mar 27, 2025
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Happens When Acids And Bases Are Mixed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
