What Holds Atoms Together In A Molecule
Muz Play
Mar 25, 2025 · 7 min read
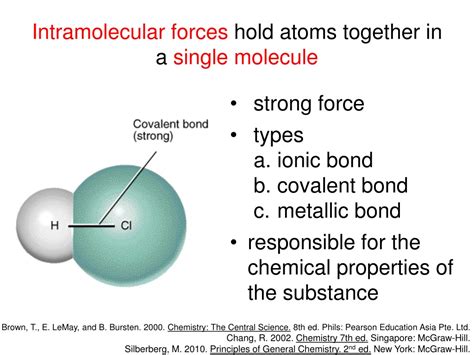
Table of Contents
What Holds Atoms Together in a Molecule? A Deep Dive into Chemical Bonding
Understanding what holds atoms together in a molecule is fundamental to grasping the essence of chemistry. It's the bedrock upon which our understanding of matter's properties, reactions, and the very fabric of the universe is built. This seemingly simple question opens a fascinating world of subatomic forces and intricate interactions. This article will explore the various types of chemical bonds, delving into the nuances of each and explaining how they govern the behavior of molecules.
The Dance of Electrons: The Key to Chemical Bonding
At the heart of chemical bonding lies the behavior of electrons, specifically those residing in the outermost electron shell, known as valence electrons. These electrons are crucial because they are the ones most readily involved in interactions with other atoms. Atoms strive for stability, and this stability is often achieved by achieving a full valence shell, mimicking the electron configuration of noble gases (Group 18 elements). This drive for stability is the driving force behind chemical bonding.
There are primarily three major types of chemical bonds:
-
Ionic Bonds: These bonds involve the transfer of electrons from one atom to another. This transfer results in the formation of ions – charged atoms. One atom loses electrons becoming positively charged (cation), while another gains electrons becoming negatively charged (anion). The electrostatic attraction between these oppositely charged ions forms the ionic bond.
-
Covalent Bonds: In contrast to ionic bonds, covalent bonds involve the sharing of electrons between atoms. This sharing allows both atoms to achieve a more stable electron configuration. Covalent bonds are typically formed between nonmetals.
-
Metallic Bonds: These bonds occur in metals and are characterized by a "sea" of delocalized electrons. The valence electrons are not associated with any particular atom but are free to move throughout the metallic structure. This delocalization accounts for many of the characteristic properties of metals, such as conductivity and malleability.
A Deeper Look at Ionic Bonding
Ionic bonds are characterized by a significant difference in electronegativity between the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A large electronegativity difference leads to the complete transfer of electrons, forming ions.
How it works: Consider the formation of sodium chloride (NaCl), common table salt. Sodium (Na) has one valence electron, while chlorine (Cl) has seven. Sodium readily loses its valence electron to achieve a stable octet (eight electrons in its valence shell), becoming a positively charged sodium ion (Na⁺). Chlorine readily accepts this electron, completing its octet and becoming a negatively charged chloride ion (Cl⁻). The strong electrostatic attraction between the positively charged Na⁺ and the negatively charged Cl⁻ ions forms the ionic bond, resulting in a stable crystal lattice structure.
Properties of Ionic Compounds: Ionic compounds typically exhibit high melting and boiling points due to the strong electrostatic forces between ions. They are often brittle and crystalline in structure. When dissolved in water, they conduct electricity because the ions are free to move and carry charge.
Factors Affecting Ionic Bond Strength
Several factors influence the strength of an ionic bond:
-
Charge of ions: Higher charges on the ions lead to stronger electrostatic attraction and thus a stronger bond. For example, MgO (magnesium oxide) with Mg²⁺ and O²⁻ ions has a stronger ionic bond than NaCl.
-
Size of ions: Smaller ions lead to stronger bonds because the electrostatic forces are inversely proportional to the distance between the ions. Smaller ions are closer together, resulting in stronger attraction.
-
Lattice energy: Lattice energy is the energy released when gaseous ions combine to form a solid ionic compound. A higher lattice energy indicates a stronger ionic bond.
Unveiling the Secrets of Covalent Bonding
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. This sharing occurs when the electronegativity difference between the atoms is small.
Types of Covalent Bonds:
-
Single Covalent Bond: Involves the sharing of one pair of electrons. For example, in a hydrogen molecule (H₂), each hydrogen atom shares one electron with the other, resulting in a single covalent bond.
-
Double Covalent Bond: Involves the sharing of two pairs of electrons. For example, in an oxygen molecule (O₂), each oxygen atom shares two electrons with the other, resulting in a double covalent bond.
-
Triple Covalent Bond: Involves the sharing of three pairs of electrons. For example, in a nitrogen molecule (N₂), each nitrogen atom shares three electrons with the other, resulting in a triple covalent bond.
Polar and Nonpolar Covalent Bonds:
The nature of a covalent bond can be further categorized as polar or nonpolar.
-
Nonpolar Covalent Bonds: Occur when the electrons are shared equally between the atoms. This usually happens when the atoms have similar electronegativities. Examples include H₂, Cl₂, and O₂.
-
Polar Covalent Bonds: Occur when the electrons are shared unequally between the atoms. This arises due to a difference in electronegativity. The atom with higher electronegativity attracts the shared electrons more strongly, resulting in a partial negative charge (δ⁻) on that atom and a partial positive charge (δ⁺) on the other atom. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
Factors Influencing Covalent Bond Strength
Several factors affect the strength of a covalent bond:
-
Bond order: Higher bond order (single, double, triple) corresponds to a stronger bond due to increased electron density between the atoms.
-
Bond length: Shorter bond lengths generally indicate stronger bonds because the atoms are closer together.
-
Atomic size: Smaller atoms generally form stronger covalent bonds because the shared electrons are closer to the nuclei.
Exploring Metallic Bonding: A Sea of Electrons
Metallic bonding is unique and responsible for many characteristic properties of metals. In metals, the valence electrons are delocalized, meaning they are not associated with any particular atom but are free to move throughout the entire metallic structure. This "sea" of delocalized electrons acts as a glue, holding the metal atoms together.
Properties of Metals:
The delocalized electrons explain several key properties of metals:
-
High electrical conductivity: The free-flowing electrons can readily carry an electric current.
-
High thermal conductivity: The electrons can efficiently transfer heat throughout the metal.
-
Malleability and ductility: The metallic structure can be deformed without breaking because the delocalized electrons can easily adjust to the changes in the arrangement of metal atoms.
Beyond the Basics: Intermolecular Forces
While chemical bonds hold atoms together within a molecule, intermolecular forces are the attractions between molecules. These forces are weaker than chemical bonds but play a crucial role in determining the physical properties of substances, such as boiling point, melting point, and solubility. Examples include:
-
London Dispersion Forces: These are weak forces that arise due to temporary fluctuations in electron distribution around an atom or molecule. They are present in all molecules.
-
Dipole-Dipole Forces: These forces occur between polar molecules that have permanent dipoles. The positive end of one molecule attracts the negative end of another molecule.
-
Hydrogen Bonding: A special type of dipole-dipole force that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. Hydrogen bonds are relatively strong intermolecular forces.
Conclusion: A Unified View of Chemical Bonding
The forces that hold atoms together in a molecule are multifaceted and dictate a vast array of physical and chemical properties. Understanding the nature of ionic, covalent, and metallic bonds, along with intermolecular forces, is fundamental to comprehending the behavior of matter at a molecular level. From the simple salt crystal to the complex structures of biological macromolecules, the interplay of these forces shapes the world around us. This exploration serves as a springboard for further investigation into the intricate and beautiful world of chemical bonding and its profound implications across various scientific disciplines.
Latest Posts
Latest Posts
-
How To Do Bohr Rutherford Diagrams
May 12, 2025
-
Is Milk Pure Substance Or Mixture
May 12, 2025
-
Power Series Of 1 1 X
May 12, 2025
-
Is Boron Trifluoride Polar Or Nonpolar
May 12, 2025
-
Which Point Of The Beam Experiences The Most Compression
May 12, 2025
Related Post
Thank you for visiting our website which covers about What Holds Atoms Together In A Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.