What Holds The Hydrogen Atoms To The Oxygen Atom
Muz Play
Apr 01, 2025 · 5 min read
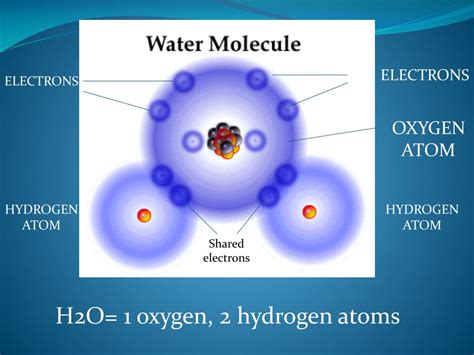
Table of Contents
What Holds Hydrogen Atoms to Oxygen Atoms? Unveiling the Secrets of the Covalent Bond in Water
Water, H₂O, the elixir of life, a ubiquitous substance covering most of our planet's surface. Its simple chemical formula belies the incredible complexity of the bonds that hold it together. Understanding what holds hydrogen atoms to oxygen atoms is key to understanding the unique properties of water, from its high boiling point to its ability to act as a universal solvent. This journey delves into the fascinating world of chemical bonding, focusing specifically on the covalent bond and the intricacies of the water molecule.
The Electromagnetic Dance: Understanding Chemical Bonds
At the heart of the matter lies the electromagnetic force. Atoms are composed of a positively charged nucleus (containing protons and neutrons) and negatively charged electrons orbiting this nucleus. Chemical bonds arise from the interplay of these charges. Atoms strive for stability, often achieved by filling their outermost electron shell (valence shell) with electrons. This drive for stability dictates how atoms interact and form bonds.
There are several types of chemical bonds, each characterized by how atoms share or transfer electrons:
Ionic Bonds: The Transfer of Electrons
Ionic bonds occur when one atom transfers one or more electrons to another atom. This transfer results in the formation of ions: positively charged cations (the atom that lost electrons) and negatively charged anions (the atom that gained electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond. Table salt (NaCl) is a classic example: sodium (Na) loses an electron to chlorine (Cl), forming Na⁺ and Cl⁻ ions, which are then held together by the ionic bond.
Covalent Bonds: The Sharing of Electrons
In contrast to ionic bonds, covalent bonds involve the sharing of electrons between atoms. This sharing allows both atoms to achieve a more stable electron configuration. The shared electrons are attracted to the nuclei of both atoms, creating a bond that holds them together. Covalent bonds are typically found between non-metal atoms, and they are the type of bond that holds hydrogen atoms to oxygen atoms in water.
The Covalent Bond in Water: A Deeper Dive
The water molecule (H₂O) is a prime example of a molecule formed through covalent bonds. Oxygen (O) has six electrons in its valence shell, needing two more to achieve a stable octet (eight electrons). Each hydrogen (H) atom has one electron in its valence shell, needing one more to achieve a stable duet (two electrons).
The oxygen atom achieves its octet by sharing one electron with each of the two hydrogen atoms. Simultaneously, each hydrogen atom achieves its duet by sharing its electron with the oxygen atom. This sharing of electrons forms two covalent bonds, holding the two hydrogen atoms to the single oxygen atom.
Polarity: Unequal Sharing
While the covalent bond in water involves electron sharing, it's not an equal sharing. Oxygen is more electronegative than hydrogen. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Oxygen's higher electronegativity means it attracts the shared electrons more strongly than hydrogen. This unequal sharing creates a polar covalent bond, resulting in a slightly negative charge (δ-) on the oxygen atom and slightly positive charges (δ+) on the hydrogen atoms.
This polarity is crucial to the unique properties of water. The slightly positive hydrogen atoms are attracted to the slightly negative oxygen atoms of other water molecules, leading to hydrogen bonding.
Hydrogen Bonding: The Water Molecule's Secret Weapon
Hydrogen bonding is a special type of intermolecular force, a force of attraction between molecules, not within a molecule like a covalent bond. It occurs between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. In water, the slightly positive hydrogen atom of one water molecule is attracted to the slightly negative oxygen atom of another water molecule.
Hydrogen bonds are weaker than covalent bonds, but they are collectively strong enough to significantly influence the properties of water. They are responsible for:
-
High Boiling Point: Water has an unusually high boiling point compared to other molecules of similar size. This is because the hydrogen bonds between water molecules require considerable energy to break, leading to a higher boiling point.
-
High Surface Tension: Hydrogen bonding creates a strong cohesive force between water molecules, resulting in high surface tension. This allows water striders to walk on water and contributes to capillary action in plants.
-
High Specific Heat Capacity: Water can absorb a significant amount of heat without a large temperature change. This is due to the energy required to break hydrogen bonds, making water an excellent temperature regulator.
-
Excellent Solvent: The polar nature of water and its ability to form hydrogen bonds make it an excellent solvent for many ionic and polar substances. The positive and negative poles of water molecules can surround and dissolve charged particles, leading to its role as a universal solvent in biological systems.
Beyond the Basics: Orbital Hybridization and Molecular Geometry
To gain a more complete understanding of the bond in water, it's essential to consider the concept of orbital hybridization and molecular geometry. The oxygen atom in water utilizes its sp³ hybrid orbitals to form covalent bonds with the two hydrogen atoms. This hybridization results in a tetrahedral electron geometry, though the molecular geometry (the arrangement of atoms) is bent due to the lone pairs of electrons on the oxygen atom. These lone pairs exert repulsive forces on the bonding pairs, causing the bent shape. This bent shape contributes to the polar nature of the water molecule and its ability to form hydrogen bonds.
Conclusion: A Symphony of Forces
The seemingly simple bond holding hydrogen atoms to oxygen atoms in water is a complex interplay of electromagnetic forces, involving covalent bonding, electronegativity, polarity, and hydrogen bonding. These interactions aren't independent; they work in concert to create a molecule with unique and essential properties. Understanding these forces is crucial not just for appreciating the wonder of water but also for comprehending the fundamental principles of chemistry and its impact on life as we know it. The seemingly simple H₂O molecule is a testament to the intricate dance of atoms and the powerful forces that shape our world. From the smallest scale of atomic interactions to the vastness of oceans, the bond in water is a fundamental cornerstone of our existence.
Latest Posts
Latest Posts
-
What Is The Tension In The Rope
Apr 02, 2025
-
What Is The Magnification Of The Microscope
Apr 02, 2025
-
Is Sugar A Pure Substance Or A Mixture
Apr 02, 2025
-
Orange Juice Is Acid Or Base
Apr 02, 2025
-
When Does Segregation Occur In Meiosis
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Holds The Hydrogen Atoms To The Oxygen Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
