What Is A Conjugated Pi System
Muz Play
Mar 31, 2025 · 7 min read
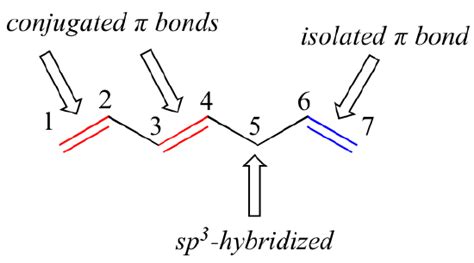
Table of Contents
What is a Conjugated Pi System? A Deep Dive into Delocalization
Conjugated pi systems are fundamental to organic chemistry, impacting the properties and reactivity of countless molecules. Understanding them is crucial for comprehending the behavior of everything from vibrant dyes and efficient solar cells to the complex structures found in nature. This comprehensive guide will explore conjugated pi systems in detail, covering their definition, characteristics, examples, and significant applications.
Defining Conjugated Pi Systems
A conjugated pi system is a system of connected p-orbitals with delocalized electrons. This delocalization is the key characteristic that differentiates conjugated systems from isolated pi systems. Let's break down this definition further:
-
Pi (π) Orbitals: These are molecular orbitals formed by the sideways overlap of p-orbitals on adjacent atoms. They are involved in double or triple bonds.
-
Connected p-orbitals: In a conjugated system, the p-orbitals are connected continuously, forming a continuous chain or network. This connection allows for electron delocalization. This is in contrast to isolated pi systems where the double bonds are separated by single bonds containing only sigma bonds.
-
Delocalized electrons: Unlike electrons in localized bonds, electrons in a conjugated system are not confined to a single bond but are spread out over the entire conjugated network. This delocalization significantly influences the molecule's properties. The electrons are said to be shared amongst the atoms participating in the conjugated system.
Key Characteristics of Conjugated Pi Systems
The delocalization of electrons in a conjugated pi system leads to a number of important characteristics:
-
Increased Stability: Delocalization lowers the overall energy of the molecule, making it more stable than its non-conjugated counterpart. This extra stability is often referred to as resonance stabilization.
-
Altered Bond Lengths: The bond lengths in a conjugated system are often intermediate between the lengths of single and double bonds. This is because the electrons are shared between all the atoms participating in the delocalization, leading to a partial double bond character throughout the entire system.
-
Unique Spectroscopic Properties: Conjugated systems often absorb light in the ultraviolet (UV) or visible (Vis) region of the electromagnetic spectrum. This absorption is responsible for the color of many organic compounds, such as dyes and pigments. The wavelength of light absorbed depends on the extent of conjugation (longer conjugated systems absorb at longer wavelengths). This is a key feature used in analytical chemistry to determine the presence of conjugated systems using UV-Vis Spectroscopy.
-
Enhanced Reactivity: The presence of delocalized electrons makes conjugated systems more susceptible to certain chemical reactions, particularly electrophilic aromatic substitution and other reactions involving electron-rich pi systems.
-
Planarity (Generally): For effective conjugation, the p-orbitals need to be able to overlap efficiently. Therefore, conjugated systems are generally planar or nearly planar. However, there are exceptions such as twisted conjugated systems which can exhibit special properties.
Examples of Conjugated Pi Systems
Numerous organic molecules possess conjugated pi systems. Here are some illustrative examples:
-
Benzene (C₆H₆): The classic example of a conjugated system. The six carbon atoms in benzene form a ring with alternating single and double bonds. However, the electrons are delocalized across all six carbon atoms, resulting in a particularly stable aromatic ring.
-
1,3-Butadiene (C₄H₆): A simple linear conjugated diene with two double bonds separated by a single bond. The four carbon p-orbitals overlap to form a conjugated pi system.
-
Polyenes: Long chains of carbon atoms with alternating single and double bonds (e.g., polyacetylene). These have extended conjugation, resulting in significant properties like electrical conductivity.
-
Polycyclic Aromatic Hydrocarbons (PAHs): Molecules containing multiple fused benzene rings (e.g., naphthalene, anthracene). These possess extensive delocalized pi systems.
-
Porphyrins: Cyclic tetrapyrroles, forming the core structure of heme in hemoglobin and chlorophyll in plants. The extensive conjugated system is vital for their function.
-
Carotenoids: Naturally occurring pigments found in plants and some animals, responsible for many of their colors. Their extensive conjugation contributes to their color and light-harvesting properties.
-
Cyanine Dyes: A class of synthetic dyes with a conjugated polymethine chain, exhibiting intense color and finding applications in photography and biological staining.
Factors Affecting Conjugation
Several factors influence the extent and effectiveness of conjugation:
-
Spacial Arrangement: Planarity is crucial. If the p-orbitals are twisted out of alignment, overlap is reduced, and the conjugation is weakened. Steric hindrance can sometimes prevent perfect planarity.
-
Presence of Heteroatoms: Conjugation can involve heteroatoms (atoms other than carbon) with lone pairs of electrons that can participate in the delocalized pi system. Examples include nitrogen, oxygen, and sulfur in various heterocyclic compounds.
-
Bond Alternation: Alternating single and double bonds are a hallmark of conjugation. However, the presence of other electron-withdrawing or electron-donating groups can also affect the degree of conjugation and charge distribution within the system.
-
Chain Length: The length of the conjugated system significantly affects its properties. Longer chains lead to greater delocalization, lower energy levels, and absorption of longer wavelengths of light.
Applications of Conjugated Pi Systems
Conjugated pi systems are crucial in many areas of science and technology:
-
Dyes and Pigments: The ability of conjugated systems to absorb visible light makes them ideal for creating colored materials used in textiles, paints, and cosmetics. The color can be precisely tuned by manipulating the length and structure of the conjugated system.
-
Organic Electronics: Conjugated polymers and oligomers are used in organic light-emitting diodes (OLEDs), organic photovoltaic cells (OPVs), and organic field-effect transistors (OFETs), offering flexible and potentially low-cost alternatives to traditional silicon-based electronics.
-
Nonlinear Optics: Conjugated systems can exhibit nonlinear optical properties, meaning their response to light is not proportional to the intensity of the light. This finds applications in optical signal processing and optical data storage.
-
Medicine: Many biologically active molecules contain conjugated pi systems. For instance, many drugs and natural products rely on their conjugated systems for their activity. Understanding the relationship between structure and activity is a major focus of medicinal chemistry.
-
Catalysis: Conjugated systems are involved in many catalytic processes, both homogeneous and heterogeneous. Their ability to readily accept and donate electrons makes them effective in redox reactions.
-
Materials Science: Conjugated systems are used to create advanced materials with unique properties, such as conductivity, strength, and flexibility. These materials find application in various fields, including aerospace and construction.
Beyond Simple Conjugation: Advanced Concepts
While the basic principles of conjugation are relatively straightforward, more advanced concepts explore the nuances and complexities of these systems:
-
Cross-Conjugation: This refers to systems where conjugated segments are connected through a single bond, but the pi systems aren't directly linked. The degree of delocalization is less than in fully conjugated systems.
-
Extended Conjugation: This refers to very long conjugated systems, often found in polymers. These can exhibit unique properties like conductivity and semiconductivity.
-
Aromaticity: A special type of conjugation found in cyclic systems satisfying Hückel's rule (4n+2 pi electrons, where n is an integer). This imparts exceptional stability and reactivity.
-
Hyperconjugation: A type of interaction between a sigma bond (typically C-H) and an adjacent empty or partially filled p-orbital. While not strictly pi-conjugation, it contributes to stability and influences molecular properties.
-
Through-Space Conjugation: Conjugation can occur through space, even without direct bonding, if the p-orbitals are sufficiently close together.
Conclusion
Conjugated pi systems are far more than a simple concept in organic chemistry. Their inherent ability to delocalize electrons leads to a unique array of properties, impacting stability, reactivity, and spectroscopic behavior. Understanding these systems is crucial for designing and developing new materials, understanding biological processes, and advancing various fields of science and technology. The continued research into the intricacies of conjugated pi systems promises to yield further advancements in the future. Further exploration of the topics mentioned above, coupled with practical applications and further studies, will enhance your understanding of this crucial area of organic chemistry.
Latest Posts
Latest Posts
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
-
Is The Organic Layer On The Top Or Bottom
Apr 02, 2025
-
In A Solution It Is Dissolving Medium
Apr 02, 2025
-
How To Find Moles Of Naoh Used In Titration
Apr 02, 2025
-
Different Conformations Of The Same Compound
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is A Conjugated Pi System . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
