What Is A Hot Plate Used For In Chemistry
Muz Play
Mar 31, 2025 · 6 min read
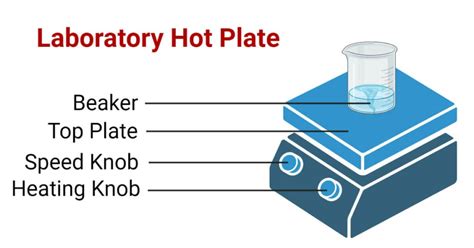
Table of Contents
What is a Hot Plate Used For in Chemistry? A Comprehensive Guide
Hot plates are ubiquitous in chemistry labs, serving as indispensable tools for a wide range of applications. Understanding their uses, safety precautions, and limitations is crucial for any chemist, from student to seasoned professional. This comprehensive guide delves into the multifaceted roles of hot plates in the chemical world, exploring their specific applications, advantages, and the necessary safety considerations.
The Fundamentals: What is a Hot Plate?
A hot plate, in its simplest form, is a heating device that uses electricity to generate heat. It features a flat, usually ceramic or metallic, surface that heats up evenly when electricity is applied. The temperature is typically adjustable, allowing for precise control over the heating process. While seemingly simple, hot plates are vital pieces of equipment, providing a controlled and consistent heat source for various chemical procedures. They are distinct from Bunsen burners, which provide an open flame, offering a safer and more controlled heating method for many applications.
Diverse Applications of Hot Plates in Chemistry
Hot plates find utility in an incredibly wide spectrum of chemical processes. Their applications span diverse areas of chemistry, including:
1. Heating Solutions and Reactions:
This is perhaps the most common application. Hot plates are used to gently heat solutions, facilitating the dissolution of solids, accelerating reaction rates, and driving off volatile components. The controlled heating allows chemists to prevent overheating, bumping, and other unwanted side reactions.
-
Dissolving Solids: Many chemical reactions require dissolving solids in solvents. A hot plate provides a controlled way to increase the rate of dissolution, especially for compounds with low solubility at room temperature. This is crucial in preparing solutions for titrations, spectroscopy, and other analytical techniques.
-
Accelerating Reaction Rates: Many chemical reactions proceed faster at higher temperatures. A hot plate allows chemists to precisely control the reaction temperature, optimizing the reaction rate while preventing unwanted decomposition or side reactions. This is essential in synthetic chemistry where yield and purity are paramount.
-
Solvent Evaporation: Hot plates, often paired with a magnetic stirrer, are used to gently evaporate solvents from solutions. This is a common step in purification procedures, concentrating samples, and preparing dry solids for analysis. The controlled heating minimizes the risk of bumping and loss of sample.
2. Melting Point Determination:
While dedicated melting point apparatus exists, hot plates can be used in conjunction with a thermometer and a capillary tube to determine the melting point of solid samples. This is a fundamental technique for characterizing and identifying unknown compounds. Although less precise than dedicated equipment, this method is useful for quick estimations.
3. Heating Reaction Vessels:
Hot plates are commonly used to heat a variety of reaction vessels, including beakers, flasks, and test tubes. Using appropriate supports and clamps, the chemist can maintain a stable and even heat distribution across the reaction vessel. The controlled heating ensures uniform reaction conditions, contributing to consistent results.
4. Sterilization:
In some instances, hot plates can be used for simple sterilization processes. Heating glassware or other laboratory equipment to a specific temperature can help eliminate microorganisms, although this is not as effective as autoclaving for thorough sterilization.
5. Supporting Other Equipment:
Hot plates can provide a stable and heated platform for other instruments, such as rotary evaporators or heating mantles, enhancing the overall effectiveness and safety of the experimental setup.
Advantages of Using Hot Plates
The widespread use of hot plates in chemistry labs is driven by several key advantages:
-
Precise Temperature Control: Most hot plates offer a wide range of adjustable temperatures, enabling precise control over the heating process. This is critical for many chemical reactions which are sensitive to temperature variations.
-
Safety: Compared to open flames (like Bunsen burners), hot plates are significantly safer. They eliminate the risk of fire hazards associated with flammable solvents. The absence of an open flame reduces the risk of burns and accidental ignition.
-
Even Heat Distribution: Good quality hot plates provide uniform heat distribution across the heating surface, ensuring consistent heating of reaction vessels. This leads to more reproducible results in chemical experiments.
-
Ease of Use: Hot plates are relatively simple to operate and maintain. Their straightforward design minimizes the learning curve, making them accessible to students and researchers alike.
-
Versatility: Hot plates can be used with a wide range of reaction vessels and experimental setups, making them incredibly versatile tools in the chemical laboratory.
-
Cost-Effectiveness: Hot plates are generally less expensive than more specialized heating equipment, making them a cost-effective solution for many laboratory needs.
Safety Precautions when Using Hot Plates
Despite their advantages, safety remains paramount when using hot plates in a chemistry lab. Several precautions must be consistently followed:
-
Use Appropriate Heat-Resistant Materials: Only use glassware and other equipment designed for high temperatures. Avoid using plastic or other heat-sensitive materials near the hot plate.
-
Never Leave a Hot Plate Unattended: Always supervise the hot plate while it is in operation. The risk of overheating or accidental spills is significant if left unattended.
-
Use Proper Support Stands: Securely clamp or support glassware on a stable stand to prevent tipping or breakage. This minimizes the risk of spills and potential burns.
-
Handle Hot Glassware with Care: Use appropriate tongs or gloves to handle hot glassware after heating to avoid burns. Hot glassware does not always appear hot to the touch.
-
Turn Off the Hot Plate After Use: Always turn off the hot plate and allow it to cool completely before cleaning or storing. This minimizes the risk of burns and extends the lifespan of the equipment.
-
Wear Appropriate Personal Protective Equipment (PPE): Always wear appropriate safety glasses and gloves when working with hot plates and chemicals. Additional PPE may be necessary depending on the specific chemicals being handled.
-
Be Aware of Flammable Materials: Keep flammable solvents away from the hot plate. The heat generated could ignite these materials, leading to a serious fire hazard.
-
Proper Ventilation: Ensure adequate ventilation in the laboratory to prevent the buildup of harmful fumes or gases produced during heating. A fume hood should be used whenever necessary.
Limitations of Hot Plates
While hot plates are invaluable tools, they do have certain limitations:
-
Maximum Temperature Limitations: Most hot plates have a maximum temperature limit. For reactions requiring temperatures beyond this limit, alternative heating methods such as heating mantles or oil baths might be necessary.
-
Uneven Heating at High Temperatures: At very high temperatures, some hot plates may exhibit uneven heat distribution, potentially affecting the accuracy and reproducibility of the experiment.
-
Inability to Heat Large Volumes: Hot plates may not be suitable for heating large volumes of liquids due to potential overheating or uneven heating. Larger-scale heating may require different techniques.
-
Potential for Hot Spots: While generally providing even heating, hot spots can sometimes occur, especially on older or poorly maintained hot plates. Careful monitoring of temperature is crucial to prevent localized overheating.
Conclusion: Hot Plates – Essential Tools in the Chemist's Arsenal
Hot plates represent indispensable tools in virtually every chemistry laboratory. Their ability to provide a safe, controlled, and consistent heat source makes them suitable for a vast array of applications, from simple heating of solutions to more complex chemical reactions and analyses. However, understanding their limitations and adhering strictly to safety protocols are paramount for ensuring successful experiments and maintaining a safe working environment. By mastering the techniques and precautions associated with hot plate usage, chemists can confidently leverage this fundamental instrument to drive their research forward.
Latest Posts
Latest Posts
-
For Most Substances Solubility Is Blank As Temperature
Apr 02, 2025
-
Differentiate Between Systemic And Pulmonary Circulation
Apr 02, 2025
-
Graph Of Atomic Number Vs Atomic Radius
Apr 02, 2025
-
Name The Four Social Change Theories
Apr 02, 2025
-
Which Of These Infectious Agents Do Not Have Nucleic Acid
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is A Hot Plate Used For In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
