What Is A Polar Protic Solvent
Muz Play
Apr 01, 2025 · 6 min read
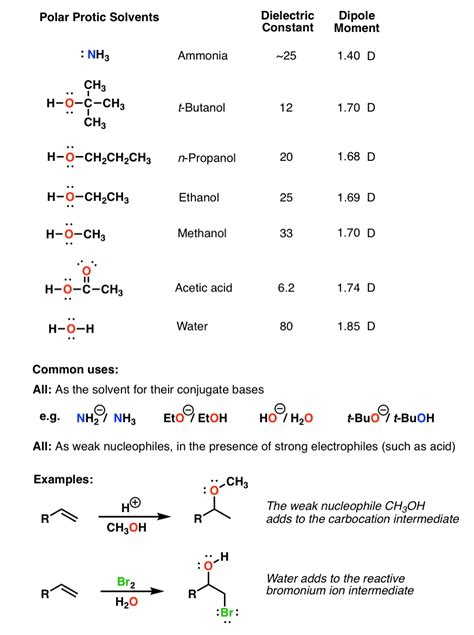
Table of Contents
What is a Polar Protic Solvent? A Deep Dive into Properties, Examples, and Applications
Polar protic solvents are a crucial component in many chemical reactions and processes. Understanding their properties and how they interact with solutes is key to mastering organic chemistry and many other chemical disciplines. This comprehensive guide will delve into the world of polar protic solvents, exploring their characteristics, providing numerous examples, and highlighting their wide-ranging applications.
Understanding Polarity and Proticity
Before we dive into the specifics of polar protic solvents, let's clarify the terms "polar" and "protic."
Polarity: The Uneven Distribution of Charge
Polarity refers to the distribution of electrical charge within a molecule. In polar molecules, the electrons are not shared equally between atoms, resulting in a partial positive charge (δ+) on one end and a partial negative charge (δ-) on the other. This uneven distribution creates a dipole moment. The greater the difference in electronegativity between the atoms, the more polar the molecule will be. Water (H₂O) is a classic example of a highly polar molecule.
Proticity: The Presence of Hydrogen Bonding
Proticity describes the ability of a solvent to donate hydrogen bonds. A protic solvent contains an O-H or N-H bond that can donate a proton (H⁺) to a solute. This hydrogen bonding significantly impacts the solvent's ability to dissolve and interact with other molecules.
Polar Protic Solvents: The Combination
A polar protic solvent combines both characteristics: it possesses a significant dipole moment and has the ability to form hydrogen bonds. This unique combination leads to specific solvation properties, making these solvents ideal for certain reactions and applications but less suitable for others.
Key Properties of Polar Protic Solvents
The combined polarity and proticity of these solvents give rise to several key properties:
-
High Dielectric Constant: The high dielectric constant helps to stabilize ions and charged species in solution, making them excellent solvents for ionic compounds and reactions involving charged intermediates. This is because the solvent molecules can effectively shield the charges from each other, reducing the electrostatic forces between them.
-
Hydrogen Bond Donation and Acceptance: Their ability to both donate and accept hydrogen bonds plays a crucial role in solvating polar molecules and influencing reaction rates and mechanisms. Hydrogen bonding can significantly stabilize transition states, thereby affecting reaction kinetics.
-
Good Solvents for Polar Compounds: Polar protic solvents readily dissolve polar molecules like alcohols, sugars, and amino acids. The hydrogen bonding interactions between the solvent and solute molecules are the driving force behind this solubility.
-
Can Participate in Reactions: Some polar protic solvents can actively participate in reactions, acting as reactants or catalysts. This is particularly relevant in acid-base reactions, where the solvent can donate or accept protons.
-
Viscosity: Many polar protic solvents exhibit higher viscosity compared to their aprotic counterparts. This higher viscosity can affect reaction rates and mass transfer processes.
Examples of Polar Protic Solvents
Many common solvents fall into the polar protic category. Here are some prominent examples:
-
Water (H₂O): The most ubiquitous polar protic solvent. Its exceptional ability to dissolve a wide range of substances makes it invaluable in various applications.
-
Methanol (CH₃OH): A relatively simple alcohol, methanol is a good solvent for many organic compounds and is commonly used in chemical synthesis and as a fuel.
-
Ethanol (CH₃CH₂OH): Similar to methanol, ethanol is a widely used solvent in various industries, including pharmaceuticals and beverages.
-
Formic Acid (HCOOH): A stronger acid than many other polar protic solvents, formic acid finds use in various chemical processes and as a preservative.
-
Acetic Acid (CH₃COOH): A weaker acid than formic acid, acetic acid is widely used as a solvent in chemical synthesis and as a component in many industrial processes.
-
Ammonia (NH₃): While less commonly used as a solvent compared to others on this list, liquid ammonia is a powerful polar protic solvent used in specific applications.
-
Hydrogen Fluoride (HF): A highly corrosive and dangerous solvent, hydrogen fluoride is exceptionally polar and protic, making it useful in specific specialized applications despite its hazards.
-
Water-Alcohol Mixtures: Mixtures of water with alcohols like methanol or ethanol are often employed to fine-tune the solvent properties for specific applications, creating a spectrum of polarity and proticity.
Applications of Polar Protic Solvents
The unique properties of polar protic solvents make them indispensable across numerous applications:
-
Chemical Synthesis: They are extensively used in various chemical reactions, acting as solvents, reactants, or catalysts. Reactions such as SN1 nucleophilic substitutions and esterification often favor polar protic solvents.
-
Pharmaceutical Industry: Polar protic solvents are crucial in the production and formulation of many pharmaceuticals. They are used as solvents for dissolving active pharmaceutical ingredients, as reaction media, and in formulation processes.
-
Biochemistry and Biology: Water, the most prevalent polar protic solvent, plays an essential role in all biological systems. Other polar protic solvents are used in biochemical research and experiments involving biological molecules.
-
Analytical Chemistry: Polar protic solvents are often used as mobile phases in chromatography, allowing for the separation and analysis of various compounds.
-
Industrial Processes: Numerous industrial processes rely on polar protic solvents. Examples include cleaning, extraction, and various manufacturing processes.
Comparing Polar Protic Solvents with Other Solvent Types
To fully understand the significance of polar protic solvents, it's helpful to compare them with other types of solvents:
Polar Aprotic Solvents
These solvents are polar but lack the ability to donate hydrogen bonds. Examples include acetone, dimethyl sulfoxide (DMSO), and acetonitrile. Polar aprotic solvents are often preferred for reactions where strong hydrogen bonding could interfere with the reaction mechanism. They are less likely to participate actively in the reaction itself.
Nonpolar Solvents
These solvents have little to no dipole moment and do not form hydrogen bonds. Examples include hexane, benzene, and toluene. Nonpolar solvents are generally used for dissolving nonpolar substances. They are unsuitable for reactions involving charged species or those requiring strong solvent-solute interactions.
Choosing the Right Polar Protic Solvent
Selecting the appropriate polar protic solvent for a specific application involves careful consideration of several factors:
-
Solubility: The solvent must effectively dissolve the reactants and products.
-
Reactivity: The solvent should not interfere with the desired reaction.
-
Safety: Consider the toxicity, flammability, and other safety hazards associated with the solvent.
-
Cost: The cost-effectiveness of the solvent should be considered.
-
Environmental impact: Choose a solvent with minimal environmental impact.
Conclusion: The Versatility of Polar Protic Solvents
Polar protic solvents are a diverse class of compounds with wide-ranging applications in chemistry, biology, and industry. Their unique combination of polarity and proticity leads to specific solvation properties that make them suitable for various reactions and processes. Understanding the properties and characteristics of polar protic solvents is fundamental to mastering many aspects of chemistry and related disciplines. Choosing the correct polar protic solvent for a particular application requires careful consideration of factors such as solubility, reactivity, safety, cost, and environmental impact. The versatility and importance of these solvents cannot be overstated. They remain essential tools in the chemist's arsenal for various applications across multiple industries. Further research into the fine-tuning of properties through solvent mixtures and novel polar protic solvents will continue to shape various fields in the future.
Latest Posts
Latest Posts
-
Naming Ionic Compounds With Transition Metals
Apr 02, 2025
-
The Subunits Of A Triglyceride Are
Apr 02, 2025
-
Write An Equation Of The Line In Standard Form
Apr 02, 2025
-
How Does Carbon Dioxide Enter The Leaf
Apr 02, 2025
-
Arrhenius Acid Vs Bronsted Lowry Acid
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is A Polar Protic Solvent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
