What Is A Property Of A Solid
Muz Play
Mar 21, 2025 · 6 min read
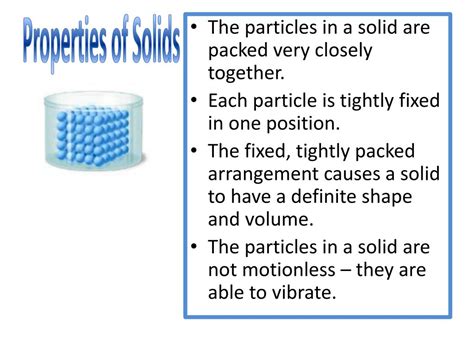
Table of Contents
What is a Property of a Solid? A Deep Dive into the Characteristics of Solids
Solids are one of the four fundamental states of matter, alongside liquids, gases, and plasmas. Understanding the properties of solids is crucial in numerous scientific fields, from materials science and engineering to chemistry and physics. This comprehensive article will explore the diverse properties of solids, categorizing them and delving into the underlying reasons behind their behavior.
Defining the Properties of Solids: A Multifaceted Perspective
The properties of a solid are the characteristics that define its physical and chemical behavior. These properties can be broadly classified into several categories:
1. Physical Properties: Observable Characteristics
Physical properties are characteristics that can be observed or measured without changing the chemical composition of the solid. These include:
-
Shape and Volume: Solids possess a definite shape and volume. Unlike liquids and gases, they do not readily conform to the shape of their container. This is due to the strong intermolecular forces holding their constituent particles together in a fixed arrangement.
-
Density: Density is a measure of mass per unit volume. Solids generally have high densities compared to liquids and gases due to the close packing of their particles. However, density varies significantly depending on the material; for example, the density of osmium is much higher than that of balsa wood.
-
Melting Point: The melting point is the temperature at which a solid transforms into a liquid. This transition occurs when the thermal energy overcomes the intermolecular forces holding the solid structure together. The melting point is a characteristic property of a solid and is often used for identification purposes.
-
Boiling Point: While not directly a property of the solid state itself, the boiling point of the liquid phase derived from the solid provides indirect information about the intermolecular forces within the solid. Stronger intermolecular forces result in higher boiling points.
-
Hardness: Hardness refers to a solid's resistance to scratching or indentation. It is a measure of the strength of the bonds between the constituent particles. Diamonds, for example, possess exceptionally high hardness due to the strong covalent bonds between carbon atoms.
-
Brittleness: Brittleness describes a solid's tendency to fracture or shatter under stress. This is often related to the type of bonding and the crystalline structure of the material.
-
Malleability and Ductility: Malleability is the ability of a solid to be deformed under compressive stress, typically by hammering or rolling, without fracturing. Ductility is the ability to be drawn into wires under tensile stress. These properties are particularly relevant for metals.
-
Elasticity: Elasticity refers to a solid's ability to return to its original shape and size after being deformed by an external force. This property is related to the strength of the intermolecular forces and the arrangement of atoms within the solid.
-
Conductivity: Conductivity refers to a solid's ability to conduct heat and/or electricity. Metals are excellent conductors due to the presence of freely moving electrons. Insulators, on the other hand, have tightly bound electrons and offer high resistance to the flow of electricity.
-
Color: The color of a solid is determined by its interaction with light. This interaction depends on the electronic structure of the material and can range from transparent to opaque, exhibiting a wide spectrum of colors.
2. Chemical Properties: Reactivity and Transformations
Chemical properties describe how a solid interacts with other substances, leading to a change in its chemical composition. These include:
-
Reactivity: Reactivity describes a solid's tendency to undergo chemical reactions with other substances. Some solids are highly reactive, readily participating in chemical processes, while others are inert, showing little tendency to react.
-
Combustibility: Combustibility refers to a solid's ability to burn in the presence of oxygen or other oxidants. The ease of combustion depends on the chemical composition of the solid and its bonding characteristics.
-
Stability: Stability refers to a solid's resistance to decomposition or change under various conditions, such as temperature, pressure, or exposure to other chemicals.
-
Corrosion Resistance: Corrosion resistance describes a solid's ability to withstand degradation due to chemical reactions with its environment, such as oxidation or acid attack. This is a crucial property for materials used in outdoor applications or harsh environments.
Deeper Dive into Specific Solid Properties
Let's explore some key properties in more detail:
Crystalline Structure and Its Influence
The arrangement of atoms, ions, or molecules in a solid significantly influences its properties. Crystalline solids have a highly ordered, repeating three-dimensional arrangement of particles, forming a crystal lattice. This ordered structure leads to distinct properties such as anisotropy (different properties in different directions) and cleavage (tendency to break along specific planes). Examples include diamonds, quartz, and table salt.
In contrast, amorphous solids lack a long-range ordered structure. Their particles are arranged randomly, resulting in isotropic properties (same properties in all directions) and a lack of sharp melting points. Examples include glass, rubber, and many plastics.
Intermolecular Forces and Their Role
The strength of the intermolecular forces between the constituent particles of a solid greatly impacts its physical properties. Stronger intermolecular forces generally lead to:
- Higher melting and boiling points: More energy is required to overcome the strong attractions.
- Greater hardness and strength: The solid is more resistant to deformation.
- Lower compressibility: The particles are tightly packed together.
Different types of intermolecular forces exist, including:
- Ionic bonds: Electrostatic attraction between oppositely charged ions (e.g., NaCl).
- Covalent bonds: Sharing of electron pairs between atoms (e.g., diamond).
- Metallic bonds: Delocalized electrons shared among a lattice of metal atoms (e.g., copper).
- Van der Waals forces: Weak attractions between molecules (e.g., in many organic solids).
The Influence of Defects on Solid Properties
Crystalline defects, or imperfections in the crystal lattice, significantly impact the properties of solids. These defects can include:
- Point defects: Missing atoms (vacancies), extra atoms (interstitials), or substituted atoms.
- Line defects (dislocations): Disruptions in the regular arrangement of atoms along a line.
- Planar defects (grain boundaries): Interfaces between different crystal orientations.
These defects can alter mechanical strength, conductivity, and reactivity. For instance, dislocations can make a material more ductile, while grain boundaries can affect its strength and corrosion resistance.
Applications Based on Solid Properties
The diverse properties of solids are exploited in countless applications across various industries:
- Construction: Strong, durable solids like concrete, steel, and bricks are essential for building structures.
- Electronics: Semiconductors, insulators, and conductors are crucial components in electronic devices.
- Biomedical engineering: Biocompatible materials with specific mechanical and chemical properties are used in implants and prosthetics.
- Manufacturing: Materials with specific malleability, ductility, and hardness are selected for different manufacturing processes.
- Energy: Solids are used in energy storage (batteries), energy conversion (solar cells), and nuclear reactors.
Conclusion: A Multifaceted Realm
The properties of solids are a rich and multifaceted area of study. Understanding these properties is crucial for developing new materials with tailored characteristics for various applications. From the strength of steel to the conductivity of silicon, the properties of solids are fundamental to our modern world. Further research into the relationship between structure, bonding, and properties will continue to drive innovation and technological advancements across diverse fields. The information provided here serves as a comprehensive starting point for exploring this captivating realm of scientific investigation. Further research into specific materials and their unique properties will yield an even deeper understanding of this critical aspect of the physical world.
Latest Posts
Latest Posts
-
What Are The Final Products Of Meiosis
Mar 22, 2025
-
1 Name Two Ecological Roles Of Fungi
Mar 22, 2025
-
Is Burning Gas A Chemical Change
Mar 22, 2025
-
Diagram Of A Stars Life Cycle
Mar 22, 2025
-
Is Luster A Metal Or Nonmetal
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is A Property Of A Solid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
