Is Burning Gas A Chemical Change
Muz Play
Mar 22, 2025 · 5 min read
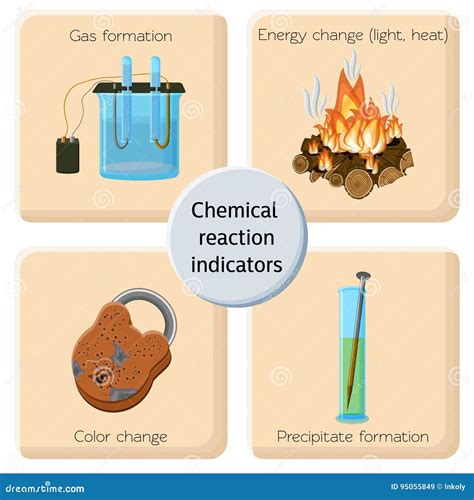
Table of Contents
Is Burning Gas a Chemical Change? A Comprehensive Look at Combustion
Burning gas, a process we rely on daily for heating, cooking, and transportation, is undeniably a chemical change. This isn't just a simple physical transformation like melting ice; it's a fundamental alteration of the molecular structure of the gas involved, resulting in entirely new substances. This article delves deep into the science behind burning gas, examining the chemical reactions, observable evidence, and the implications of this chemical process.
Understanding Chemical Changes
Before we dive into the specifics of burning gas, let's establish a clear understanding of what constitutes a chemical change. A chemical change, also known as a chemical reaction, involves the rearrangement of atoms to form new molecules with different properties. This rearrangement is irreversible – you can't simply reverse the process to get back the original substances. Key indicators of a chemical change include:
- Formation of a new substance: The resulting product(s) have distinct physical and chemical properties from the reactants.
- Change in color: A noticeable shift in color often indicates a chemical reaction.
- Release or absorption of heat: Many chemical changes involve either the release (exothermic) or absorption (endothermic) of energy.
- Production of gas: The formation of bubbles or a noticeable odor can signal a chemical reaction.
- Formation of a precipitate: A solid that forms from a solution is a clear indicator of a chemical change.
The Chemistry of Burning Gas: Combustion
Burning gas is essentially a rapid combustion reaction. Combustion is a chemical process that occurs when a substance reacts rapidly with an oxidant, usually oxygen, to produce heat and light. In the case of burning gas, the fuel (the gas) reacts with oxygen in the air, releasing significant energy in the form of heat and light.
Types of Gas Used in Combustion
Different types of gases are used as fuel in combustion processes, each with its own chemical composition and properties. Common examples include:
- Natural Gas: Primarily composed of methane (CH₄), natural gas is a widely used fuel for heating and cooking. Its combustion reaction is: CH₄ + 2O₂ → CO₂ + 2H₂O + heat + light
- Propane (C₃H₈): Used in gas grills, portable stoves, and some vehicles, propane undergoes a similar combustion reaction: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O + heat + light
- Butane (C₄H₁₀): Another common fuel found in lighters and some camping stoves, butane also combusts readily: 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O + heat + light
Evidence of Chemical Change in Burning Gas
The combustion of gas provides ample evidence supporting its classification as a chemical change:
- Heat and Light Production: The most obvious indicator is the release of heat and light. This energy release is a hallmark of exothermic chemical reactions.
- Formation of New Substances: Carbon dioxide (CO₂) and water (H₂O) are the primary products of burning most common gases. These are entirely different substances from the original gas and oxygen.
- Irreversibility: You cannot simply cool the carbon dioxide and water to magically reform the original gas and oxygen. The process is irreversible, another key characteristic of a chemical change.
- Change in Chemical Composition: The chemical composition of the fuel has fundamentally changed. The methane, propane, or butane molecules have been broken down and rearranged into new molecules. This alteration in the arrangement of atoms signifies a chemical change.
Factors Affecting Combustion
Several factors influence the efficiency and completeness of gas combustion:
- Oxygen Supply: Sufficient oxygen is crucial for complete combustion. Limited oxygen leads to incomplete combustion, producing carbon monoxide (CO), a highly toxic gas, instead of carbon dioxide.
- Fuel-to-Air Ratio: The proper ratio of fuel to air is essential for optimal combustion. An excess of fuel or air can lead to inefficient burning and potentially harmful byproducts.
- Temperature: A certain ignition temperature is needed to initiate combustion. Once ignited, the reaction generates enough heat to sustain itself.
- Pressure: Pressure affects the density of the gas mixture, influencing the rate of reaction.
Environmental Implications
The combustion of gas, while providing essential energy, also has environmental consequences. The primary concern is the release of greenhouse gases, particularly carbon dioxide. The accumulation of CO₂ in the atmosphere contributes to climate change. Incomplete combustion also releases other pollutants like carbon monoxide, particulate matter, and nitrogen oxides, which negatively impact air quality and human health.
Safety Precautions
Handling and using combustible gases requires strict adherence to safety protocols to prevent accidents. These include:
- Proper Ventilation: Adequate ventilation is crucial to prevent the buildup of harmful gases like carbon monoxide.
- Leak Detection: Regular checks for gas leaks are necessary to avoid potential fires or explosions.
- Storage and Handling: Gases should be stored and handled according to the manufacturer's instructions to minimize risks.
- Emergency Procedures: Familiarity with emergency procedures in case of gas leaks or fires is crucial.
Alternatives to Gas Combustion
Given the environmental concerns associated with gas combustion, research and development focus on cleaner energy alternatives:
- Renewable Energy Sources: Solar, wind, and hydroelectric power offer sustainable alternatives to fossil fuels.
- Hydrogen Fuel Cells: Hydrogen fuel cells produce electricity through a chemical reaction between hydrogen and oxygen, releasing only water as a byproduct.
- Biofuels: Biofuels, derived from biomass, offer a more sustainable fuel source compared to fossil fuels.
Conclusion: Burning Gas is a Definite Chemical Change
In conclusion, burning gas is unequivocally a chemical change. The process involves a fundamental alteration of the molecular structure of the gas, leading to the formation of new substances, release of energy, and the exhibition of several other characteristics indicative of chemical reactions. While gas combustion provides essential energy for various applications, it's crucial to acknowledge its environmental implications and explore cleaner energy alternatives for a sustainable future. Understanding the chemistry behind this process empowers us to utilize gas safely and responsibly while actively seeking solutions to mitigate its impact on our planet. The clear evidence of new substance formation, heat release, and irreversibility solidify its status as a definitive chemical change, a fundamental concept in chemistry and a process with significant implications for our society and environment. Continued research and technological advancements are pivotal in navigating the complexities of energy production and consumption, striking a balance between energy needs and environmental stewardship.
Latest Posts
Latest Posts
-
Determine Whether The Graph Is The Graph Of A Function
Mar 22, 2025
-
Lewis Structure Practice Worksheet And Answers
Mar 22, 2025
-
The Cell The Basic Unit Of Life
Mar 22, 2025
-
Power Series Solution Of Ordinary Differential Equations
Mar 22, 2025
-
How Many Electrons Does Li2 Have
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is Burning Gas A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
