How Many Electrons Does Li2 Have
Muz Play
Mar 22, 2025 · 5 min read
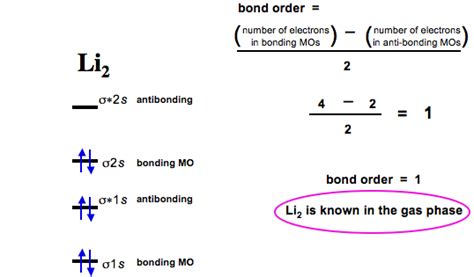
Table of Contents
How Many Electrons Does Li₂ Have? Delving into the World of Diatomic Lithium
The seemingly simple question, "How many electrons does Li₂ have?" opens a fascinating door into the world of chemical bonding, molecular orbitals, and the behavior of alkali metals. While the answer might seem straightforward at first glance, a deeper understanding requires exploring the fundamental principles of atomic structure and molecular formation. This article will delve into this question, providing a comprehensive explanation accessible to both beginners and those with a more advanced understanding of chemistry.
Understanding Lithium's Atomic Structure
Before tackling the diatomic molecule Li₂, let's establish a solid foundation by examining the atomic structure of lithium (Li). Lithium, with an atomic number of 3, possesses three protons in its nucleus and, in its neutral state, three electrons orbiting the nucleus. These electrons are distributed across energy levels, with two electrons occupying the lowest energy level (1s orbital) and one electron residing in the higher energy level (2s orbital). This electron configuration (1s²2s¹) is crucial in understanding lithium's reactivity and bonding behavior. The single electron in the 2s orbital is relatively loosely held and readily participates in chemical bonding.
The Formation of Diatomic Lithium (Li₂)
Unlike many elements that exist as stable single atoms, lithium, like other alkali metals, exists as a diatomic molecule under standard conditions, meaning it forms a molecule composed of two lithium atoms bonded together – Li₂. This bonding arises from the interaction of the valence electrons – the electrons in the outermost energy level – of the two lithium atoms.
The Bonding Process:
The formation of Li₂ involves the overlap of the 2s atomic orbitals from each lithium atom. This overlap leads to the creation of two molecular orbitals: a bonding molecular orbital (σ<sub>2s</sub>) and an antibonding molecular orbital (σ<sub>2s</sub>*). The bonding molecular orbital is lower in energy than the individual atomic orbitals, while the antibonding molecular orbital is higher in energy.
According to the aufbau principle, electrons fill the lower energy molecular orbitals first. The two valence electrons (one from each lithium atom) occupy the bonding molecular orbital (σ<sub>2s</sub>). This results in a stable diatomic molecule because the electrons are attracted to both nuclei, creating a net attractive force that holds the two lithium atoms together.
Electron Count in Li₂
With this understanding of the bonding process, we can now definitively answer the question: Li₂ has a total of six electrons. This is simply the sum of the three electrons from each of the two lithium atoms. These six electrons are distributed within the molecular orbitals, with two electrons residing in the bonding σ<sub>2s</sub> orbital and the remaining four electrons distributed into other orbitals.
Beyond the Basic Electron Count: A Deeper Dive into Molecular Orbital Theory
While the simple answer of six electrons is correct, a deeper analysis using molecular orbital theory offers a more nuanced understanding. This theory provides a framework for understanding the electronic structure of molecules and their properties.
Molecular Orbital Diagram for Li₂:
A molecular orbital diagram visually represents the energy levels and electron occupancy of the molecular orbitals. For Li₂, a simplified diagram would show the two 2s atomic orbitals combining to form a bonding σ<sub>2s</sub> orbital and an antibonding σ<sub>2s</sub>* orbital. The two valence electrons occupy the lower-energy bonding orbital. The 1s orbitals remain essentially unchanged, contributing minimally to the bonding.
Bond Order and Stability:
The bond order is a crucial concept in understanding the stability of a molecule. It's calculated as half the difference between the number of electrons in bonding orbitals and the number of electrons in antibonding orbitals. For Li₂, the bond order is (2 - 0) / 2 = 1. A bond order of 1 indicates a single covalent bond between the two lithium atoms, explaining the diatomic nature of lithium. A higher bond order generally implies a stronger and more stable bond.
Experimental Evidence and Li₂'s Properties
The existence and properties of Li₂ have been experimentally verified. Spectroscopic techniques, such as photoelectron spectroscopy, provide evidence for the molecular orbitals and electronic structure predicted by molecular orbital theory. Furthermore, the relatively weak bond in Li₂ is consistent with its low boiling point and the reactivity of alkali metals.
Applications and Significance of Understanding Li₂
Understanding the electronic structure and bonding in Li₂ is not just an academic exercise; it has practical implications. This knowledge is fundamental to comprehending:
- Alkali Metal Chemistry: The behavior of lithium and other alkali metals is directly linked to their electronic configurations and the ease with which they form bonds.
- Materials Science: Understanding diatomic lithium's properties allows for better design and application of materials containing lithium, including batteries and other electrochemical devices.
- Spectroscopy: The interaction of light with Li₂ molecules is used in spectroscopic analysis, crucial for chemical identification and characterization.
- Theoretical Chemistry: Li₂, being a relatively simple molecule, serves as a model system for testing and refining computational methods used to study more complex molecules and materials.
Expanding the Knowledge: Comparing Li₂ to other Diatomic Molecules
Comparing Li₂ to other diatomic molecules, such as H₂, O₂, and N₂, reveals the diversity in bonding and properties. H₂, with only two electrons, forms a single bond, but its bond strength is significantly higher than Li₂ due to the closer proximity of the nuclei. O₂ and N₂, with more electrons and more complex bonding, display paramagnetism and stronger triple bonds respectively, highlighting the effect of electron count and orbital interactions on molecular properties.
Conclusion: The Significance of Six Electrons in Li₂
In conclusion, Li₂ possesses a total of six electrons, a fact that directly stems from the contribution of three electrons from each lithium atom. While the simple answer might appear straightforward, the underlying principles of atomic and molecular structure provide a far richer understanding of this diatomic molecule's formation, stability, and properties. The exploration of Li₂ serves as a gateway to a deeper appreciation of chemical bonding, molecular orbital theory, and the vast landscape of chemical science. The knowledge gained from studying such simple systems provides a critical foundation for tackling the complexities of more elaborate molecules and materials. The six electrons in Li₂ are not just a number; they represent the fundamental building blocks of chemical interaction and the foundation for understanding the world around us.
Latest Posts
Latest Posts
-
4 2 Practice Solving Systems Using Substitution
Mar 22, 2025
-
The Basic Structural Unit Of The Body Is The
Mar 22, 2025
-
How To Do Mole To Mole Conversions
Mar 22, 2025
-
What Is The Purpose Of Punnett Square
Mar 22, 2025
-
Which Is Released During The Formation Of A Peptide Bond
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Li2 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
