How To Do Mole To Mole Conversions
Muz Play
Mar 22, 2025 · 6 min read
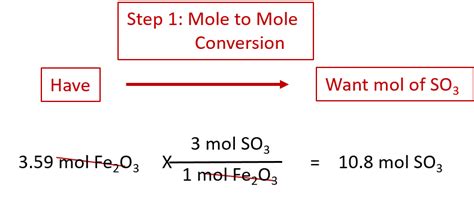
Table of Contents
How to Ace Mole to Mole Conversions: A Comprehensive Guide
Mole to mole conversions are a cornerstone of stoichiometry, a crucial area in chemistry. Mastering this skill is essential for understanding chemical reactions and predicting the amounts of reactants and products involved. While it might seem daunting at first, with a systematic approach and a solid understanding of the underlying principles, mole to mole conversions become surprisingly straightforward. This comprehensive guide will equip you with the knowledge and tools to confidently tackle these calculations.
Understanding the Mole Concept
Before diving into conversions, let's solidify our grasp on the mole. The mole (mol) is a fundamental unit in chemistry, representing a specific number of particles – Avogadro's number, approximately 6.022 x 10<sup>23</sup>. This number applies to atoms, molecules, ions, or any other specified entity. Think of it like a dozen: a dozen eggs contains 12 eggs, and a mole of carbon atoms contains 6.022 x 10<sup>23</sup> carbon atoms.
The mole's significance lies in its ability to connect the microscopic world of atoms and molecules to the macroscopic world of measurable quantities like mass and volume. This connection is facilitated through molar mass, which represents the mass of one mole of a substance in grams. For instance, the molar mass of carbon (C) is approximately 12.01 g/mol, meaning one mole of carbon atoms weighs 12.01 grams.
The Importance of Balanced Chemical Equations
The key to accurate mole to mole conversions lies in the balanced chemical equation. This equation represents a chemical reaction, showing the reactants (starting materials) on the left side and the products (resulting substances) on the right side. Crucially, the equation is balanced, meaning the number of atoms of each element is equal on both sides. This balance reflects the law of conservation of mass, stating that matter cannot be created or destroyed in a chemical reaction.
Example: Consider the combustion of methane (CH<sub>4</sub>):
CH<sub>4</sub> + 2O<sub>2</sub> → CO<sub>2</sub> + 2H<sub>2</sub>O
This equation shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. Importantly, it also tells us that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water. This is the crucial link that allows us to perform mole to mole conversions.
Performing Mole to Mole Conversions: A Step-by-Step Approach
The process of mole to mole conversion is essentially a ratio problem, using the coefficients from the balanced chemical equation as conversion factors. Here's a detailed step-by-step approach:
Step 1: Write and Balance the Chemical Equation
This is the foundational step. Ensure your chemical equation accurately represents the reaction and is completely balanced. Any error here will propagate through the entire calculation.
Step 2: Identify the Given and Desired Moles
Determine the number of moles of the substance you're starting with (the given) and the substance you want to find the number of moles for (the desired).
Step 3: Set Up the Conversion Factor
Use the coefficients from the balanced equation to create a conversion factor. The coefficient of the desired substance goes in the numerator, and the coefficient of the given substance goes in the denominator. This creates a ratio representing the molar relationship between the two substances.
Step 4: Perform the Calculation
Multiply the given moles by the conversion factor to obtain the moles of the desired substance.
Example: Let's use the methane combustion equation from earlier. If we start with 3.0 moles of methane (CH<sub>4</sub>), how many moles of water (H<sub>2</sub>O) will be produced?
-
Balanced Equation: CH<sub>4</sub> + 2O<sub>2</sub> → CO<sub>2</sub> + 2H<sub>2</sub>O
-
Given and Desired: Given: 3.0 moles CH<sub>4</sub>; Desired: moles H<sub>2</sub>O
-
Conversion Factor: From the balanced equation, the ratio of H<sub>2</sub>O to CH<sub>4</sub> is 2:1. Therefore, the conversion factor is (2 moles H<sub>2</sub>O / 1 mole CH<sub>4</sub>).
-
Calculation:
3.0 moles CH<sub>4</sub> × (2 moles H<sub>2</sub>O / 1 mole CH<sub>4</sub>) = 6.0 moles H<sub>2</sub>O
Therefore, 6.0 moles of water will be produced.
Tackling More Complex Scenarios
While the basic approach remains consistent, some scenarios might present slight variations:
1. Limiting Reactants: When multiple reactants are involved, one reactant will be completely consumed before the others. This reactant is the limiting reactant, and it dictates the amount of product formed. In such cases, you'll need to perform mole to mole conversions for each reactant to determine which is limiting and use that to calculate the amount of product.
2. Percent Yield: The theoretical yield is the amount of product predicted by stoichiometry. However, the actual yield is often less due to various factors. The percent yield accounts for this difference: (actual yield / theoretical yield) x 100%. You'll use mole to mole conversions to determine the theoretical yield before calculating the percent yield.
3. Multiple Steps: Some reactions occur in multiple steps. You'll need to perform a series of mole to mole conversions, using the product of one step as the reactant in the next.
Mastering Mole to Mole Conversions: Tips and Tricks
- Practice, Practice, Practice: The best way to master mole to mole conversions is to work through numerous problems. Start with simple examples and gradually increase the complexity.
- Visualize the Reaction: Drawing a diagram of the reaction can help visualize the relationships between reactants and products.
- Check Your Units: Always ensure your units cancel out correctly during the calculations. This is a crucial check for accuracy.
- Use Dimensional Analysis: Dimensional analysis, also known as the factor-label method, is a powerful tool for ensuring your calculations are set up correctly.
- Seek Help When Needed: Don't hesitate to ask your teacher, professor, or tutor for help if you're struggling with a particular problem.
Conclusion
Mole to mole conversions are a fundamental skill in chemistry, enabling us to understand and predict the quantitative aspects of chemical reactions. By mastering the step-by-step approach outlined in this guide and practicing regularly, you'll build a strong foundation in stoichiometry and unlock a deeper understanding of the chemical world. Remember, consistent practice and a clear understanding of the underlying principles are the keys to success. So, grab your pen and paper, and start practicing! You've got this!
Latest Posts
Latest Posts
-
How To Calculate Binding Energy Per Nucleon
Mar 23, 2025
-
What Are The Products Of This Chemical Reaction
Mar 23, 2025
-
A Neutral Atom Has Equal Numbers Of Blank And Electrons
Mar 23, 2025
-
Environmental Factors That Affect Microbial Growth
Mar 23, 2025
-
General Chemistry Principles And Modern Applications
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How To Do Mole To Mole Conversions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
