What Are The Products Of This Chemical Reaction
Muz Play
Mar 23, 2025 · 5 min read
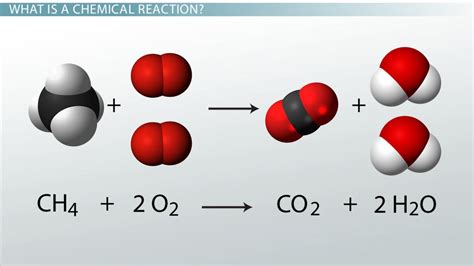
Table of Contents
What Are the Products of This Chemical Reaction? A Comprehensive Guide
Determining the products of a chemical reaction is fundamental to chemistry. It's the culmination of understanding reactants, reaction mechanisms, and the principles governing chemical transformations. This in-depth guide will explore various aspects of predicting and identifying reaction products, covering different reaction types and the factors influencing them. We'll delve into practical examples and provide tools to help you master this crucial chemical concept.
Understanding Chemical Reactions and Their Products
A chemical reaction involves the rearrangement of atoms and molecules, leading to the formation of new substances with different properties. The starting materials are called reactants, and the resulting substances are called products. The process is typically represented by a chemical equation:
Reactants → Products
The arrow indicates the direction of the reaction, and the equation must be balanced to adhere to the law of conservation of mass – the total mass of reactants equals the total mass of products.
Factors Influencing Reaction Products
Several factors can significantly influence the products formed in a chemical reaction:
- Type of reactants: The nature of the reacting substances dictates the possible pathways and products. For instance, reactions involving strong acids differ drastically from those involving organic compounds.
- Reaction conditions: Temperature, pressure, presence of catalysts, and the solvent used can all alter the reaction pathway and the resulting products. High temperatures might favor certain pathways, while catalysts can accelerate specific reactions and influence product selectivity.
- Reaction mechanism: The step-by-step process by which a reaction occurs determines the intermediate species and ultimately the final products. Understanding reaction mechanisms is crucial for predicting product formation accurately.
- Stoichiometry: The relative amounts of reactants affect the extent of the reaction and the yield of each product. Limiting reactants can dictate the overall amount of product formed.
- Equilibrium: Many reactions are reversible, reaching a state of equilibrium where the rate of the forward reaction equals the rate of the reverse reaction. The equilibrium constant determines the relative amounts of reactants and products at equilibrium.
Types of Chemical Reactions and Their Products
Chemical reactions can be broadly classified into several types, each characterized by specific patterns of product formation:
1. Synthesis Reactions (Combination Reactions):
In synthesis reactions, two or more simple substances combine to form a more complex substance.
A + B → AB
Example: The reaction between hydrogen and oxygen to form water:
2H₂ + O₂ → 2H₂O
Here, hydrogen and oxygen (reactants) combine to form water (product).
2. Decomposition Reactions:
Decomposition reactions are the reverse of synthesis reactions, where a complex substance breaks down into simpler substances.
AB → A + B
Example: The decomposition of calcium carbonate upon heating:
CaCO₃ → CaO + CO₂
Calcium carbonate decomposes into calcium oxide and carbon dioxide.
3. Single Displacement Reactions (Substitution Reactions):
In single displacement reactions, a more reactive element replaces a less reactive element in a compound.
A + BC → AC + B
Example: The reaction between zinc and hydrochloric acid:
Zn + 2HCl → ZnCl₂ + H₂
Zinc displaces hydrogen from hydrochloric acid, forming zinc chloride and hydrogen gas.
4. Double Displacement Reactions (Metathesis Reactions):
Double displacement reactions involve the exchange of ions between two compounds.
AB + CD → AD + CB
Example: The reaction between silver nitrate and sodium chloride:
AgNO₃ + NaCl → AgCl + NaNO₃
Silver nitrate and sodium chloride react to form silver chloride (a precipitate) and sodium nitrate.
5. Combustion Reactions:
Combustion reactions involve the rapid reaction of a substance with oxygen, often producing heat and light. The products typically include oxides.
Example: The combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
Methane reacts with oxygen to produce carbon dioxide and water.
6. Acid-Base Reactions (Neutralization Reactions):
Acid-base reactions involve the reaction between an acid and a base, usually producing salt and water.
HA + BOH → BA + H₂O
Example: The reaction between hydrochloric acid and sodium hydroxide:
HCl + NaOH → NaCl + H₂O
Hydrochloric acid and sodium hydroxide react to form sodium chloride (salt) and water.
7. Redox Reactions (Oxidation-Reduction Reactions):
Redox reactions involve the transfer of electrons between reactants. One reactant undergoes oxidation (loss of electrons), and another undergoes reduction (gain of electrons).
Example: The reaction between iron and copper(II) sulfate:
Fe + CuSO₄ → FeSO₄ + Cu
Iron reduces copper(II) ions, forming iron(II) sulfate and copper metal.
Predicting Reaction Products: A Systematic Approach
Predicting the products of a chemical reaction requires a systematic approach:
- Identify the reactants: Determine the chemical formulas of all the reactants involved.
- Classify the reaction type: Categorize the reaction (synthesis, decomposition, single displacement, double displacement, combustion, acid-base, redox).
- Apply the appropriate rules: Use the general rules and patterns associated with each reaction type to predict the likely products.
- Balance the equation: Ensure the equation is balanced to conserve mass.
- Consider reaction conditions: Account for factors like temperature, pressure, and catalysts that may influence the products formed.
- Consult resources: Use reference materials like textbooks, handbooks, and online databases to confirm your predictions.
Advanced Concepts and Considerations
- Organic Chemistry Reactions: Organic reactions often involve complex mechanisms and a wider array of potential products. Understanding functional groups and reaction mechanisms is crucial for predicting products in organic chemistry.
- Stereochemistry: Many reactions produce stereoisomers (molecules with the same atoms but different spatial arrangements). Predicting the stereochemistry of products requires a deep understanding of reaction mechanisms and stereoselective processes.
- Kinetics and Thermodynamics: Reaction kinetics governs the rate of a reaction, while thermodynamics determines the feasibility and equilibrium position. Both are crucial in understanding product distribution.
- Spectroscopic Techniques: Techniques like NMR, IR, and Mass Spectrometry are used to identify and confirm the structure of reaction products experimentally.
Conclusion
Determining the products of a chemical reaction is a cornerstone of chemistry. By understanding the different reaction types, the factors that influence product formation, and employing a systematic approach, you can accurately predict and identify the resulting substances. This knowledge is crucial for numerous applications in various fields, from industrial chemistry and materials science to medicine and environmental science. Remember to always consider the specific reactants, reaction conditions, and mechanisms involved for a comprehensive and accurate prediction. Continuous learning and practice are key to mastering this fundamental aspect of chemistry. Further exploration of specific reaction mechanisms and classes of compounds will enhance your abilities to predict reaction outcomes with increasing accuracy and confidence.
Latest Posts
Latest Posts
-
Moment Of Intertia Of A Rod
Mar 25, 2025
-
What Determines The Function Of A Protein
Mar 25, 2025
-
What Is Explained By The Sliding Filament Theory
Mar 25, 2025
-
Polarity Lead To Heat Of Vaporization
Mar 25, 2025
-
What Holds Atoms Together In A Molecule
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Are The Products Of This Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
