Which Is Released During The Formation Of A Peptide Bond
Muz Play
Mar 22, 2025 · 6 min read
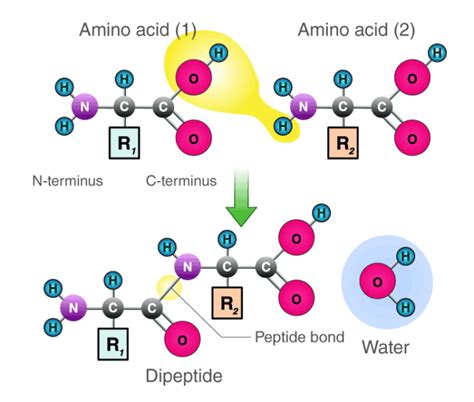
Table of Contents
Which Molecule is Released During the Formation of a Peptide Bond?
The formation of a peptide bond is a fundamental process in biochemistry, crucial for the synthesis of proteins. Understanding this process, down to the molecular level, is key to comprehending numerous biological functions. This article delves deep into the specifics of peptide bond formation, focusing on the molecule released during this vital reaction.
The Peptide Bond: A Cornerstone of Protein Structure
Proteins, the workhorses of the cell, are linear polymers of amino acids. These amino acids are linked together by a special type of covalent bond, the peptide bond, also known as an amide bond. This bond is formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another. The precise mechanism of peptide bond formation is a crucial aspect of protein biosynthesis.
The Dehydration Synthesis Reaction
Peptide bond formation is a dehydration synthesis or condensation reaction. This means that a molecule of water is removed during the bond formation process. This is a key characteristic that distinguishes it from other types of chemical reactions. Let's break down the precise steps:
Step-by-Step Mechanism:
-
Approach: The carboxyl group of one amino acid approaches the amino group of another amino acid. This approach is influenced by various factors including enzyme activity and the surrounding environment.
-
Proton Transfer: A proton (H+) is transferred from the amino group to the hydroxyl group (-OH) of the carboxyl group. This is often facilitated by an enzyme.
-
Water Molecule Formation: The hydroxyl group (-OH) and the proton (H+) combine to form a water molecule (H2O).
-
Peptide Bond Formation: With the water molecule released, the carbon atom of the carboxyl group and the nitrogen atom of the amino group form a new covalent bond – the peptide bond. This bond is a stable amide linkage.
-
Product: The resulting molecule is a dipeptide, formed by the joining of two amino acids. The process can continue, adding more amino acids to create longer polypeptide chains and eventually proteins.
The Crucial Role of Water in Peptide Bond Formation
The water molecule released during peptide bond formation is a direct consequence of the reaction mechanism. It is not merely a byproduct; its removal is essential for the creation of the stable peptide bond. The bond itself is stabilized through resonance, a phenomenon where electrons are delocalized over the peptide bond, contributing to its strength and stability.
Significance of the Dehydration Reaction:
The dehydration synthesis process is not unique to peptide bond formation. It is a fundamental type of reaction involved in the synthesis of many biological molecules, including polysaccharides (carbohydrates) and nucleic acids (DNA and RNA). The release of water provides the driving force for these condensation reactions, allowing the creation of complex macromolecules from smaller building blocks.
Enzymes and Peptide Bond Formation: Ribosomes and Peptidyl Transferases
In living organisms, the formation of peptide bonds is not a spontaneous process. It is highly regulated and catalyzed by specialized enzymes, primarily within the ribosomes, the protein synthesis machinery of cells. The key enzyme responsible for peptide bond formation is peptidyl transferase.
Peptidyl Transferase: The Catalyst
Peptidyl transferase resides within the ribosome's large subunit. It facilitates the interaction between the amino acids and precisely positions them to promote the dehydration reaction. It lowers the activation energy required for the reaction to occur, dramatically increasing its rate. Peptidyl transferase uses various strategies to align and stabilize the reactants, making peptide bond formation an efficient and accurate process.
The Ribosome's Role Beyond Catalysis:
The ribosome's role extends beyond just catalyzing peptide bond formation. It precisely reads the messenger RNA (mRNA) sequence, selecting the appropriate transfer RNA (tRNA) molecules, each carrying a specific amino acid. This ensures the accurate ordering of amino acids in the growing polypeptide chain, ultimately determining the protein's structure and function.
The Importance of Accurate Peptide Bond Formation
The accuracy of peptide bond formation is absolutely crucial for protein function. A single error in the amino acid sequence can have significant consequences, potentially leading to a non-functional or even harmful protein. The cellular mechanisms involved in protein synthesis have evolved intricate quality control measures to minimize errors in this vital process.
Quality Control Mechanisms:
These quality control measures include proofreading by the ribosome, mechanisms that ensure the correct matching of tRNA molecules to codons on the mRNA, and cellular pathways that degrade misfolded or incorrectly synthesized proteins.
Beyond Dipeptides: Building Polypeptide Chains
Once a dipeptide is formed, the process can be repeated, adding more amino acids to the chain. The carboxyl end of the dipeptide can then react with the amino group of a third amino acid, and so on. This elongation continues until the entire amino acid sequence encoded by the mRNA is translated into a complete polypeptide chain.
Peptide Bond Hydrolysis: The Reverse Reaction
While peptide bond formation is essential for protein synthesis, the reverse reaction – peptide bond hydrolysis – is equally important in protein degradation. Hydrolysis breaks the peptide bond by adding a water molecule, separating the amino acids. This process is crucial for recycling amino acids and regulating protein levels within the cell. Various enzymes, called proteases, catalyze the hydrolysis of peptide bonds.
Proteases: Regulators of Protein Turnover
Proteases are involved in a vast array of cellular processes, including protein degradation, regulation of protein activity, and immune responses. They are highly specific, targeting specific peptide bonds within a protein, allowing for precise control of protein breakdown.
Understanding Peptide Bond Formation: A Multidisciplinary Perspective
The formation of a peptide bond is a fascinating process that beautifully illustrates the intricate nature of biochemistry. It involves concepts from organic chemistry (understanding the reaction mechanism), enzymology (the role of peptidyl transferase), and molecular biology (protein synthesis). Studying this process contributes to our understanding of many critical biological processes, ranging from protein synthesis and cellular regulation to disease mechanisms and drug development.
Applications and Future Directions
Further research into the intricacies of peptide bond formation has far-reaching implications:
- Drug Discovery: Understanding the mechanism of peptide bond formation is crucial for developing drugs that target protein synthesis, either to inhibit it (e.g., in cancer treatment) or to enhance it (e.g., in treating muscle-wasting diseases).
- Biotechnology: Manipulating the process of peptide bond formation is important for various biotechnological applications, including protein engineering, the synthesis of artificial peptides, and the creation of novel biomaterials.
- Understanding Diseases: Mutations affecting the enzymes involved in peptide bond formation can lead to various genetic disorders. Research in this area is crucial for understanding the pathogenesis of these diseases and developing effective therapies.
The release of a water molecule during the formation of a peptide bond is a cornerstone of protein biosynthesis. This seemingly simple dehydration reaction is a complex process finely tuned by cellular machinery, ensuring the accurate construction of the proteins that underpin all life processes. Further exploration of this fundamental biochemical reaction holds immense promise for advancing our knowledge and developing novel technologies.
Latest Posts
Latest Posts
-
Does Electric Potential Increase With Distance
Mar 23, 2025
-
How To Calculate Binding Energy Per Nucleon
Mar 23, 2025
-
What Are The Products Of This Chemical Reaction
Mar 23, 2025
-
A Neutral Atom Has Equal Numbers Of Blank And Electrons
Mar 23, 2025
-
Environmental Factors That Affect Microbial Growth
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Which Is Released During The Formation Of A Peptide Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
