What Is A Suspension In Chemistry
Muz Play
Mar 20, 2025 · 6 min read
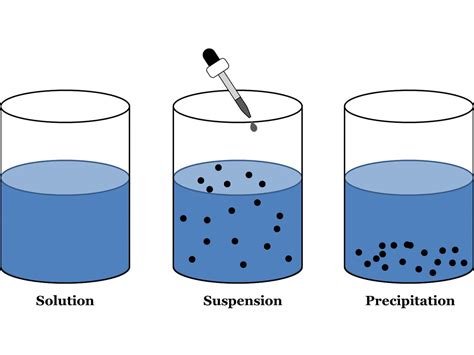
Table of Contents
What is a Suspension in Chemistry? A Comprehensive Guide
Suspensions are ubiquitous in our daily lives, from the mud stirred up in a puddle to the medications we take. Understanding suspensions in chemistry requires delving into their fundamental properties, characteristics, and applications. This comprehensive guide will explore suspensions in detail, covering everything from their definition and preparation to their uses and limitations.
Defining a Suspension in Chemistry
In chemistry, a suspension is a heterogeneous mixture containing solid particles dispersed in a liquid medium. These solid particles are relatively large, typically greater than 1 micrometer in diameter, and are not dissolved in the liquid. Unlike solutions, where the solute particles are evenly distributed at a molecular level, the particles in a suspension are visible to the naked eye and tend to settle out over time if left undisturbed. This settling is a key characteristic differentiating suspensions from other types of mixtures like colloids and solutions.
Key Characteristics of Suspensions:
- Heterogeneous Nature: Suspensions are visibly heterogeneous; you can easily distinguish the solid particles from the liquid medium.
- Particle Size: The suspended particles are relatively large, typically exceeding 1 micrometer.
- Settling: The solid particles will eventually settle at the bottom of the container if left undisturbed.
- Tyndall Effect: While suspensions don't exhibit the Tyndall effect as strongly as colloids, larger particles can scatter light, though this scattering is generally less pronounced.
- Filtration: The solid particles can be separated from the liquid medium using simple filtration techniques.
How Suspensions are Formed: Preparation Methods
The preparation of a suspension involves dispersing solid particles in a liquid medium. Several methods can achieve this, depending on the desired properties of the suspension:
1. Mechanical Dispersion:
This is the simplest method. It involves physically mixing the solid particles with the liquid using techniques like stirring, shaking, or using a homogenizer. The efficiency of this method depends on the size and nature of the solid particles and the viscosity of the liquid.
2. Wetting Agents and Stabilizers:
Often, the solid particles tend to clump together (agglomerate) due to inter-particle attractive forces. To prevent this, wetting agents and stabilizers are often added. Wetting agents reduce the surface tension between the solid particles and the liquid, facilitating better dispersion. Stabilizers, on the other hand, increase the viscosity of the suspension or provide steric hindrance, preventing the particles from settling or clumping together.
3. Controlled Precipitation:
Some suspensions are prepared by carefully controlling the precipitation of a solid from a solution. By adjusting factors like temperature, pH, and concentration, the size and distribution of the precipitated particles can be controlled to create a stable suspension.
Types of Suspensions:
Suspensions can be classified based on several factors, including the nature of the dispersed phase and the dispersing medium, the particle size distribution, and the presence of additives.
1. Based on the Dispersing Medium:
- Aqueous Suspensions: These suspensions use water as the dispersing medium. Many pharmaceutical suspensions fall under this category.
- Non-aqueous Suspensions: These employ organic liquids like oils or solvents as the dispersing medium. These are commonly used in paints and some industrial applications.
2. Based on Particle Size:
While all suspensions have particles larger than 1 micrometer, the particle size distribution can significantly impact the suspension's properties. A suspension with a narrow particle size distribution will generally be more stable than one with a wide distribution.
3. Based on Additives:
The presence of additives like wetting agents, stabilizers, and preservatives significantly affects the suspension's stability and shelf life. These additives help prevent settling, caking, and microbial growth.
Applications of Suspensions:
Suspensions find wide-ranging applications across various fields:
1. Pharmaceutical Industry:
Suspensions are a common dosage form for oral medications, particularly for those who have difficulty swallowing tablets or capsules. The suspended particles contain the active pharmaceutical ingredient, ensuring controlled drug release. Examples include antacids, antibiotics, and some vaccines.
2. Cosmetics and Personal Care Products:
Many cosmetic products, like lotions, creams, and sunscreens, are suspensions. The suspended particles provide various properties, such as opacity, texture, and controlled release of active ingredients.
3. Paints and Coatings:
Paints are classic examples of suspensions. Pigments, which provide color and opacity, are suspended in a liquid medium, typically a binder, allowing for even application and good adhesion to surfaces.
4. Food Industry:
Several food products are suspensions, including milk, chocolate drinks, and some salad dressings. The suspended particles provide texture and contribute to the overall sensory experience.
5. Agricultural Applications:
Suspensions are used in various agricultural applications, such as pesticides and herbicides. These formulations allow for uniform distribution of the active ingredients across the treated area.
6. Industrial Applications:
Suspensions are used in various industrial processes, including mineral processing, wastewater treatment, and material synthesis.
Challenges and Limitations of Suspensions:
Despite their widespread use, suspensions present several challenges:
1. Settling:
The tendency of solid particles to settle is a major limitation. This can lead to uneven distribution of the active ingredient, affecting efficacy and appearance. Stabilizers and rheology modifiers are crucial in mitigating this issue.
2. Caking:
Settled particles can sometimes form a hard cake at the bottom of the container, making redispersion difficult. This is often caused by strong inter-particle attractive forces.
3. Flocculation:
Flocculation is the aggregation of particles into larger clusters, also leading to sedimentation and instability. Careful selection of stabilizers and control of the preparation process are essential to prevent flocculation.
Factors Affecting Stability of Suspensions:
The stability of a suspension is crucial for its effectiveness and shelf life. Several factors influence the stability:
1. Particle Size and Size Distribution:
Smaller, uniformly sized particles tend to result in more stable suspensions.
2. Density Difference between Particles and Medium:
A significant density difference can accelerate sedimentation.
3. Viscosity of the Medium:
Higher viscosity slows down sedimentation and improves stability.
4. Temperature:
Temperature changes can affect the viscosity of the medium and the interaction between particles, impacting stability.
5. Electrostatic Interactions:
Electrostatic interactions between particles can either stabilize or destabilize the suspension.
6. Presence of Additives:
Wetting agents, dispersing agents, and stabilizers are critical for enhancing suspension stability.
Conclusion:
Suspensions are essential heterogeneous mixtures with wide-ranging applications across various industries. Understanding their properties, preparation methods, and the factors affecting their stability is vital for designing and utilizing suspensions effectively. From pharmaceutical formulations to industrial processes, suspensions play a crucial role in our daily lives, highlighting their significance in chemistry and beyond. This comprehensive guide provides a strong foundation for further exploration of this fascinating area of chemistry. Further research into specific types of suspensions and their unique applications will undoubtedly reveal even greater insights into the versatile nature of these mixtures.
Latest Posts
Latest Posts
-
Is Melting Point Physical Or Chemical
Mar 21, 2025
-
Are Oxidation Numbers The Same As Charges
Mar 21, 2025
-
Manifest And Latent Functions Of Education
Mar 21, 2025
-
Issuance Of Common Stock Journal Entry
Mar 21, 2025
-
Is Salt Water A Mixture Or Pure Substance
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is A Suspension In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
