What Is Delta G Double Dagger
Muz Play
Mar 21, 2025 · 6 min read
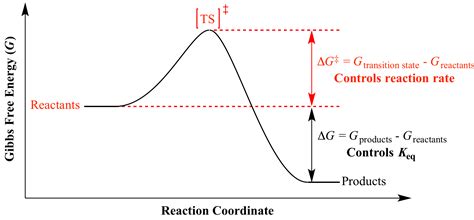
Table of Contents
What is ΔG‡ (Delta G Double Dagger)? Understanding the Gibbs Free Energy of Activation
The term "ΔG‡," often written as "delta G double dagger," represents the Gibbs Free Energy of Activation. It's a crucial concept in chemistry, particularly in chemical kinetics and enzymology, that quantifies the energy barrier a system must overcome to proceed from reactants to products. Understanding ΔG‡ is fundamental to predicting reaction rates and designing catalysts. This article provides a comprehensive explanation of ΔG‡, its significance, factors influencing it, and its applications.
What Does ΔG‡ Represent?
At a molecular level, chemical reactions don't occur instantaneously. Reactant molecules need to acquire sufficient energy to overcome an energy barrier before transforming into products. This energy barrier is the activation energy, often denoted as E<sub>a</sub>. ΔG‡ is the thermodynamic equivalent of this activation energy, representing the Gibbs Free Energy difference between the transition state and the reactants.
The transition state is a high-energy, unstable intermediate state that exists momentarily during the reaction. It's neither reactant nor product but a crucial point along the reaction coordinate. The Gibbs Free Energy of activation, ΔG‡, essentially quantifies the energy required to reach this transition state from the initial state of the reactants.
A lower ΔG‡ indicates a faster reaction rate because fewer molecules need to overcome a smaller energy hurdle. Conversely, a higher ΔG‡ signifies a slower reaction, as a larger fraction of molecules must possess sufficient energy to reach the transition state.
Connecting ΔG‡ to Reaction Rate: The Eyring Equation
The relationship between ΔG‡ and the reaction rate constant (k) is described by the Eyring equation:
k = (k<sub>B</sub>T/h) * exp(-ΔG‡/RT)
Where:
- k is the rate constant of the reaction
- k<sub>B</sub> is the Boltzmann constant
- T is the absolute temperature
- h is Planck's constant
- R is the ideal gas constant
- ΔG‡ is the Gibbs Free Energy of activation
This equation elegantly links the microscopic properties (Boltzmann and Planck constants) with macroscopic observables (temperature and reaction rate). It highlights the exponential dependence of the rate constant on ΔG‡: a small change in ΔG‡ can significantly impact the reaction rate.
Factors Affecting ΔG‡
Several factors can influence the Gibbs Free Energy of activation, ultimately determining the speed of a reaction:
1. Temperature:
Increasing the temperature increases the kinetic energy of molecules. A higher fraction of molecules then possesses the energy to surpass the activation barrier, leading to a faster reaction rate. The Eyring equation directly reflects this temperature dependence.
2. Reactant Concentration:
Higher reactant concentrations lead to more frequent collisions, increasing the probability of successful collisions that overcome the activation barrier. This effect is often incorporated into the rate law, but the underlying principle relates to the probability of reaching the transition state.
3. Catalysts:
Catalysts are substances that accelerate reaction rates without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower ΔG‡. This involves the formation of an intermediate complex with the catalyst, which subsequently decomposes into products, bypassing the high-energy transition state of the uncatalyzed reaction.
4. Solvent Effects:
The solvent plays a crucial role in the reaction, affecting the stability of both reactants and the transition state. Polar solvents, for instance, can stabilize charged transition states, lowering ΔG‡. Conversely, non-polar solvents might destabilize charged intermediates, increasing ΔG‡.
5. Steric Effects:
Bulkiness of reactant molecules can hinder their approach to the transition state, effectively raising ΔG‡. Steric hindrance makes it harder for molecules to align properly for the reaction to proceed.
6. Electronic Effects:
Electron-donating or electron-withdrawing groups in reactant molecules can significantly influence the electronic structure of the transition state, impacting its stability and, thus, ΔG‡.
ΔG‡ in Enzyme Catalysis
Enzymes are biological catalysts that dramatically accelerate reaction rates within living organisms. They achieve this by significantly lowering the ΔG‡ of the catalyzed reaction. Enzymes achieve this through several mechanisms, including:
- Proximity and Orientation Effects: Enzymes bind substrates in a specific orientation, maximizing the probability of successful collisions. This reduces the entropy contribution to ΔG‡.
- Acid-Base Catalysis: Enzyme residues can donate or accept protons, stabilizing charged intermediates in the transition state.
- Covalent Catalysis: The enzyme forms a temporary covalent bond with the substrate, creating a more stable intermediate.
- Metal Ion Catalysis: Metal ions in the enzyme's active site can participate in various catalytic mechanisms, including charge stabilization and redox reactions.
By understanding the factors that influence ΔG‡ within an enzymatic reaction, researchers can design drugs that act as enzyme inhibitors or activators, manipulating reaction rates for therapeutic purposes.
Calculating ΔG‡
ΔG‡ can't be directly measured experimentally. However, it can be calculated from the experimentally determined rate constant (k) using the Eyring equation, provided the temperature is known. This requires rearrangement of the Eyring equation to solve for ΔG‡:
ΔG‡ = -RT * ln(k h / k<sub>B</sub>T)
This calculation relies on the assumption that the transmission coefficient (κ), which represents the probability that a system crossing the transition state proceeds to products, is equal to 1. This is a reasonable approximation for many reactions, but it may not be valid in all cases.
ΔG‡ vs. ΔG° (Standard Gibbs Free Energy Change)
It's important to distinguish ΔG‡ from ΔG°, the standard Gibbs Free Energy change of a reaction. ΔG° represents the overall energy difference between reactants and products at standard conditions. ΔG° determines the equilibrium constant (K) of the reaction, indicating the relative amounts of reactants and products at equilibrium. ΔG‡, on the other hand, determines the rate at which the equilibrium is reached. A reaction can have a large negative ΔG° (thermodynamically favorable), but a high ΔG‡ (kinetically unfavorable), leading to a slow reaction.
Applications of ΔG‡
The understanding and manipulation of ΔG‡ find widespread applications in various fields:
- Drug Design: Determining ΔG‡ of enzyme-substrate interactions is crucial for designing drugs that inhibit or activate enzymes involved in diseases.
- Catalysis Research: Understanding ΔG‡ helps design more efficient catalysts for industrial processes, improving reaction yields and reducing energy consumption.
- Materials Science: Predicting reaction rates and mechanisms is essential for developing new materials with desired properties.
- Environmental Chemistry: Understanding the kinetics of environmental reactions helps to model pollutant degradation and predict their fate in the environment.
Conclusion
ΔG‡, the Gibbs Free Energy of activation, is a critical parameter quantifying the energy barrier to a chemical reaction. It is inextricably linked to the reaction rate, as described by the Eyring equation. Understanding the factors affecting ΔG‡ – temperature, concentration, catalysts, solvent, steric and electronic effects – is crucial for manipulating reaction rates and designing efficient processes in various fields, from drug discovery to materials science. While not directly measurable, ΔG‡ can be calculated from experimental rate constants, providing valuable insights into reaction mechanisms and kinetics. The distinction between ΔG‡ and ΔG° is essential to understand both the thermodynamic feasibility and the kinetic rate of a reaction. The ongoing research into the intricacies of ΔG‡ continues to expand our ability to control and optimize chemical processes.
Latest Posts
Latest Posts
-
How To Identify A Sedimentary Rock
Mar 21, 2025
-
Rolles And The Mean Value Theorem
Mar 21, 2025
-
Types Of Chemical Reactions Answer Key
Mar 21, 2025
-
Metallic Character Of Elements In Periodic Table
Mar 21, 2025
-
What Is The Serial Position Curve
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is Delta G Double Dagger . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
