What Is Kw In Chemistry Value
Muz Play
Mar 16, 2025 · 6 min read
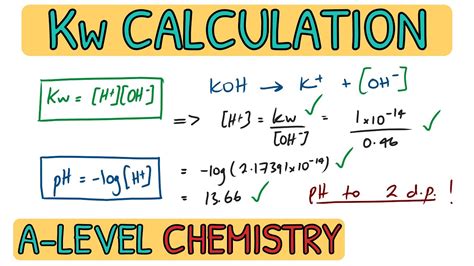
Table of Contents
What is Kw in Chemistry? Understanding the Ion Product of Water
The seemingly simple molecule of water, H₂O, plays a crucial role in countless chemical reactions. While often perceived as neutral, water actually undergoes a process called self-ionization, where water molecules react with each other to produce hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). This equilibrium reaction is fundamental to understanding acidity and basicity in aqueous solutions, and the equilibrium constant associated with it is known as Kw, the ion product of water. This article will delve into the details of Kw, its significance, how it's calculated, and its applications in various chemical contexts.
Understanding Water's Self-Ionization
Water's self-ionization is an equilibrium reaction represented as:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
This equation shows that two water molecules react to form one hydronium ion and one hydroxide ion. The hydronium ion is often simplified to H⁺ in many chemical equations, but it's important to remember that the proton (H⁺) is highly reactive and exists in aqueous solutions as a solvated ion, typically bound to a water molecule.
The equilibrium constant for this reaction is Kw, expressed as:
Kw = [H₃O⁺][OH⁻]
where:
- [H₃O⁺] represents the concentration of hydronium ions in moles per liter (mol/L or M).
- [OH⁻] represents the concentration of hydroxide ions in moles per liter (mol/L or M).
The Value of Kw and its Temperature Dependence
The value of Kw is not a constant; it is temperature dependent. At 25°C (298 K), Kw has a value of approximately 1.0 x 10⁻¹⁴. This means that at this temperature, the product of the concentrations of hydronium and hydroxide ions is always equal to 1.0 x 10⁻¹⁴. This seemingly small value reflects the fact that only a very tiny fraction of water molecules are ionized at any given time.
However, as temperature increases, the value of Kw increases. This is because higher temperatures provide more energy to overcome the energy barrier for the self-ionization reaction, leading to a greater degree of ionization and thus higher concentrations of H₃O⁺ and OH⁻ ions. The increase in Kw with temperature is not linear; it's more complex and can be described using empirical equations or graphically with data obtained from experimental measurements.
Kw at Different Temperatures: A Brief Overview
While the standard value of Kw (1.0 x 10⁻¹⁴) is used for most calculations at room temperature, it's crucial to acknowledge its temperature dependence. Here's a glimpse at how Kw changes:
- At lower temperatures (below 25°C): Kw is smaller than 1.0 x 10⁻¹⁴. The degree of water ionization is lower.
- At higher temperatures (above 25°C): Kw is larger than 1.0 x 10⁻¹⁴. The degree of water ionization is higher.
Precise values of Kw at various temperatures are available in chemical handbooks and databases. Remember to always use the appropriate Kw value for the temperature at which your experiment or calculation is conducted.
Kw and pH: The Relationship
Kw is intrinsically linked to the pH scale, which measures the acidity or basicity of a solution. The pH is defined as:
pH = -log₁₀[H₃O⁺]
Similarly, pOH is defined as:
pOH = -log₁₀[OH⁻]
Using the logarithmic properties and the equation for Kw, we can derive a crucial relationship:
pH + pOH = 14 (at 25°C)
This relationship holds true only at 25°C because Kw changes with temperature. At other temperatures, the sum of pH and pOH will differ from 14.
Applications of Kw in Chemistry
The ion product of water, Kw, is a fundamental concept used across several areas of chemistry:
1. Determining the pH and pOH of pure water and neutral solutions:
In pure water, the concentrations of H₃O⁺ and OH⁻ are equal. Therefore, at 25°C:
[H₃O⁺] = [OH⁻] = √Kw = √(1.0 x 10⁻¹⁴) = 1.0 x 10⁻⁷ M
This leads to a pH of 7 and a pOH of 7, indicating a neutral solution.
2. Calculating the pH or pOH of acidic and basic solutions:
Knowing Kw allows us to calculate the pH or pOH of a solution if the concentration of either H₃O⁺ or OH⁻ is known. For example, if we know the concentration of a strong acid, we can calculate the concentration of OH⁻ using the Kw expression and then calculate the pOH and subsequently the pH.
3. Understanding the behavior of weak acids and weak bases:
Kw plays a vital role in calculating the equilibrium concentrations of ions in solutions of weak acids and weak bases. The equilibrium expressions for these reactions involve Kw, helping us determine the extent of ionization and calculate the pH or pOH.
4. Solubility of sparingly soluble salts:
Some salts have very low solubility in water. However, their solubility can be significantly affected by the pH of the solution. Using Kw, and understanding the acid-base properties of the ions produced from the dissolution of these salts, we can determine the solubility of these sparingly soluble substances in solutions of different pH.
5. Buffer solutions:
Buffer solutions resist changes in pH when small amounts of acid or base are added. These solutions often contain a weak acid and its conjugate base or a weak base and its conjugate acid. The equilibrium between these species, governed partially by Kw, is responsible for the buffering capacity of the solution.
6. Titration calculations:
Kw helps understand and perform accurate calculations during titrations. For instance, the equivalence point of a titration can be determined by tracking changes in the concentration of H₃O⁺ or OH⁻, calculations that incorporate Kw.
Kw and its Importance in Various Chemical Fields
The significance of Kw extends far beyond basic acid-base chemistry. Its implications are felt in diverse scientific disciplines:
-
Environmental Chemistry: Kw helps in analyzing water quality, understanding the impact of pollutants, and monitoring environmental changes that can affect the acidity or alkalinity of water bodies.
-
Biochemistry: Kw plays a crucial role in understanding biochemical processes that take place in aqueous environments within living organisms. Many biological reactions are pH-sensitive, so controlling the pH within the biological range requires consideration of the principles governed by Kw.
-
Analytical Chemistry: Kw is essential in various analytical techniques, including titrations and pH measurements, contributing to the accuracy and reliability of chemical analysis.
-
Geochemistry: Understanding the self-ionization of water is crucial for analyzing geochemically relevant processes, like the weathering of rocks, that may alter the pH of groundwater.
Conclusion
The ion product of water, Kw, is a fundamental constant that underpins our understanding of acidity, basicity, and equilibrium in aqueous solutions. Its temperature dependence highlights the dynamic nature of water's self-ionization. The applications of Kw are vast, extending across multiple branches of chemistry and related scientific disciplines. A comprehensive grasp of Kw is essential for anyone pursuing studies or research in chemistry and related fields. Understanding its value and its implications helps us interpret chemical phenomena, perform accurate calculations, and solve real-world problems. Remember that the precise value of Kw must always be considered relative to the temperature under examination. This crucial detail ensures the accurate and relevant application of this fundamental constant in various chemical contexts.
Latest Posts
Latest Posts
-
What Kingdom Is A Human In
Mar 16, 2025
-
What Is The Voice Of A Small Place
Mar 16, 2025
-
Spider Crab And Algae Symbiotic Relationship
Mar 16, 2025
-
What Is A Fixed Wing Aircraft
Mar 16, 2025
-
What Is Group 17 On The Periodic Table Called
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Is Kw In Chemistry Value . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
