What Is Ph At The Equivalence Point
Muz Play
Mar 25, 2025 · 6 min read
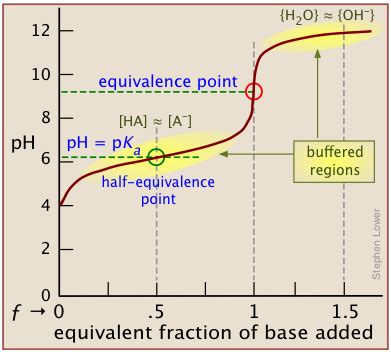
Table of Contents
What is pH at the Equivalence Point? A Comprehensive Guide
Understanding pH at the equivalence point is crucial in various fields, from analytical chemistry to environmental science. This comprehensive guide will delve deep into this concept, explaining what it is, how it's determined, and its significance in different contexts. We'll explore the factors influencing pH at the equivalence point and provide practical examples to solidify your understanding.
What is the Equivalence Point?
The equivalence point in a titration is the point at which the amount of titrant added is stoichiometrically equivalent to the amount of analyte present. In simpler terms, it's the point where the reactants have completely neutralized each other. This doesn't necessarily mean the solution is neutral (pH 7); it depends entirely on the nature of the acid and base involved.
Strong Acid-Strong Base Titration
In a strong acid-strong base titration (e.g., HCl and NaOH), the equivalence point occurs at pH 7. This is because the reaction produces water and a neutral salt. The strong acid completely dissociates, providing H⁺ ions, and the strong base completely dissociates, providing OH⁻ ions. These ions react to form water, leaving behind only the neutral salt.
Example: The titration of 0.1M HCl with 0.1M NaOH. At the equivalence point, the moles of HCl equal the moles of NaOH, resulting in a neutral solution with a pH of 7.
Weak Acid-Strong Base Titration
The scenario changes when we consider a weak acid-strong base titration (e.g., acetic acid and NaOH). Here, the equivalence point will have a pH greater than 7. This is because the conjugate base of the weak acid (acetate ion in this case) hydrolyzes water, producing hydroxide ions (OH⁻), thus increasing the solution's pH. The extent of hydrolysis depends on the strength of the weak acid; weaker acids will result in a more alkaline equivalence point.
Example: Titrating acetic acid with NaOH. At the equivalence point, the solution contains the acetate ion, which reacts with water to form acetic acid and hydroxide ions, leading to a pH above 7.
Strong Acid-Weak Base Titration
Conversely, in a strong acid-weak base titration (e.g., HCl and ammonia), the equivalence point will have a pH less than 7. This is because the conjugate acid of the weak base (ammonium ion in this case) hydrolyzes water, producing hydronium ions (H₃O⁺), thus decreasing the solution's pH. Again, the strength of the weak base determines the extent of hydrolysis and the resulting pH at the equivalence point.
Example: Titrating ammonia with HCl. At the equivalence point, the solution contains the ammonium ion, which reacts with water to form ammonia and hydronium ions, resulting in a pH below 7.
Weak Acid-Weak Base Titration
Titrations involving weak acid and weak base are more complex. The pH at the equivalence point will depend on the relative strengths of the acid and base. Predicting the exact pH without calculations can be challenging. The pH will generally be closer to 7 if the acid and base have similar strengths, but deviations are common. Calculations often involve considering the equilibrium constants of both the acid and base.
Example: Titrating acetic acid with ammonia. Predicting the pH at the equivalence point requires considering the Ka of acetic acid and the Kb of ammonia.
Determining the pH at the Equivalence Point
Determining the pH at the equivalence point involves different approaches depending on the type of titration.
Calculation Methods
For strong acid-strong base titrations, calculating the pH is straightforward. Since the products are neutral, the pH is simply 7 (at 25°C).
For weak acid-strong base and strong acid-weak base titrations, the calculation is more involved. It usually requires:
- Determining the concentration of the conjugate base/acid: This involves using stoichiometry to calculate the moles of conjugate base/acid formed at the equivalence point, then dividing by the total volume.
- Using the appropriate equilibrium constant: For weak acid-strong base titrations, use the Kb of the conjugate base. For strong acid-weak base titrations, use the Ka of the conjugate acid.
- Applying the appropriate equilibrium expression: Set up the equilibrium expression for the hydrolysis reaction and solve for the hydroxide or hydronium ion concentration.
- Calculating the pH: Use the calculated concentration of hydroxide or hydronium ions to calculate the pH or pOH, and then convert accordingly.
These calculations can be complex and often require the use of the quadratic formula or iterative methods.
Graphical Methods
Titration curves, which plot pH against the volume of titrant added, provide a visual representation of the titration process. The equivalence point is typically identified as the point of steepest slope on the curve. The pH at this point can then be read directly from the graph. This method is particularly useful when dealing with weak acids or bases where precise calculations are challenging.
Indicators
Acid-base indicators change color over a specific pH range. Choosing an indicator with a color change close to the equivalence point's pH ensures accurate determination of the endpoint, which is an approximation of the equivalence point. The endpoint and equivalence point may not be exactly the same, but a well-chosen indicator minimizes the difference.
Factors Influencing pH at the Equivalence Point
Several factors influence the pH at the equivalence point:
- Temperature: The autoionization constant of water (Kw) is temperature-dependent. Changes in temperature affect the pH of the solution at the equivalence point, even for strong acid-strong base titrations.
- Concentration of the reactants: While concentration doesn't change the theoretical pH at the equivalence point, it impacts the sharpness of the titration curve. Higher concentrations result in a sharper curve, making the equivalence point easier to determine.
- Ionic strength: High ionic strength can influence the activity coefficients of ions, slightly altering the pH at the equivalence point.
- Solvent: Using a non-aqueous solvent significantly affects the pH at the equivalence point due to changes in the solvent's acidity or basicity.
Significance of pH at the Equivalence Point
The pH at the equivalence point carries significant importance in various applications:
- Analytical Chemistry: Accurate determination of the equivalence point is essential in quantitative analysis to determine the concentration of an unknown solution.
- Environmental Monitoring: pH measurements are crucial in assessing water quality and determining the presence of pollutants. The pH at the equivalence point can help in understanding the buffering capacity of natural waters.
- Pharmaceutical Industry: pH control is vital in drug formulation and stability. The pH at the equivalence point aids in developing suitable formulations.
- Industrial Processes: Many industrial processes rely on controlled pH conditions, and the pH at the equivalence point is relevant in several applications.
Conclusion
The pH at the equivalence point is a fundamental concept in acid-base chemistry. Understanding the factors that influence this pH and the various methods for its determination is crucial for accurate analysis and control in numerous fields. While simple in concept for strong acid-strong base titrations, the calculations become more complex for weak acid-weak base or mixtures, emphasizing the importance of understanding both theoretical calculations and practical titration methods. Mastering this concept is key to proficiency in analytical chemistry and related disciplines. Furthermore, the use of accurate measurements, correct indicator selection, and proper interpretation of titration curves is paramount for successful titration experiments and reliable data.
Latest Posts
Latest Posts
-
Gramatica A The Verb Gustar Worksheet Answers
Mar 26, 2025
-
What Is The Vertical Columns On The Periodic Table Called
Mar 26, 2025
-
Nonpolar Molecules Are The Result Of Unequal Electron Pair Sharing
Mar 26, 2025
-
What Does Triangle Mean In Physics
Mar 26, 2025
-
Moment Of Inertia For A Uniform Rod
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
