What Is Polymer Of Amino Acids
Muz Play
Mar 17, 2025 · 6 min read
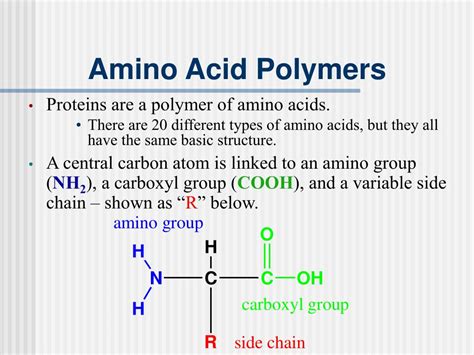
Table of Contents
What is a Polymer of Amino Acids? Delving into the World of Proteins
Proteins are the workhorses of life, essential for virtually every biological process. Understanding their structure and function is fundamental to grasping the intricacies of biology and biochemistry. At the heart of this understanding lies the answer to a crucial question: what is a polymer of amino acids? The simple answer is: a protein. Let's delve deeper into this fascinating topic, exploring the chemistry, structure, and diverse functions of these amazing biomolecules.
Amino Acids: The Building Blocks of Proteins
Before we understand protein polymers, we need to examine their fundamental building blocks: amino acids. These organic molecules are characterized by a central carbon atom (the alpha carbon) bonded to four groups:
- An amino group (-NH2): This group is basic and readily accepts protons.
- A carboxyl group (-COOH): This group is acidic and readily donates protons.
- A hydrogen atom (-H): A simple hydrogen atom.
- A side chain (R group): This is the variable group that distinguishes one amino acid from another. The R group can be as simple as a hydrogen atom (as in glycine) or a complex, branched structure (as in leucine).
There are 20 standard amino acids that are commonly incorporated into proteins during protein synthesis. These amino acids are classified based on the properties of their side chains:
- Nonpolar, aliphatic amino acids: These amino acids have hydrophobic (water-repelling) side chains. Examples include glycine, alanine, valine, leucine, isoleucine, and methionine.
- Aromatic amino acids: These amino acids have ring structures in their side chains, often contributing to the protein's absorption of ultraviolet light. Examples include phenylalanine, tyrosine, and tryptophan.
- Polar, uncharged amino acids: These amino acids have hydrophilic (water-attracting) side chains. Examples include serine, threonine, cysteine, asparagine, and glutamine.
- Positively charged amino acids (basic): These amino acids have positively charged side chains at physiological pH. Examples include lysine, arginine, and histidine.
- Negatively charged amino acids (acidic): These amino acids have negatively charged side chains at physiological pH. Examples include aspartate and glutamate.
Peptide Bonds: Linking Amino Acids Together
Amino acids are linked together via peptide bonds to form polypeptide chains. A peptide bond is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. This reaction involves the removal of a water molecule (dehydration synthesis). A chain of amino acids linked by peptide bonds is called a polypeptide.
The Peptide Bond's Characteristics
The peptide bond has several important characteristics:
- Planar: The atoms involved in the peptide bond lie in a relatively planar configuration due to resonance. This restricts rotation around the peptide bond, influencing the overall conformation of the protein.
- Partial double bond character: The resonance structure gives the peptide bond some double-bond character, making it stronger and less likely to rotate freely.
- Polarity: The peptide bond is polar, contributing to the overall polarity of the protein.
Levels of Protein Structure: From Primary to Quaternary
The structure of a protein dictates its function. Protein structure is described at four levels:
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein is simply the linear sequence of amino acids in the polypeptide chain. This sequence is dictated by the genetic code and is crucial because it determines all higher levels of structure. Even a single amino acid substitution can significantly alter the protein's function. Consider the infamous sickle cell anemia, caused by a single amino acid change in the hemoglobin protein.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to local, regular folding patterns within the polypeptide chain. These patterns are stabilized by hydrogen bonds between the backbone amide (-NH) and carbonyl (=O) groups. The most common secondary structures are:
- Alpha-helices: A right-handed coiled structure stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues further down the chain.
- Beta-sheets: Extended polypeptide chains arranged side-by-side, stabilized by hydrogen bonds between adjacent strands. Beta-sheets can be parallel (strands run in the same direction) or antiparallel (strands run in opposite directions).
- Turns and loops: These are less regular structures that connect alpha-helices and beta-sheets.
3. Tertiary Structure: The 3D Arrangement
The tertiary structure describes the overall three-dimensional arrangement of a polypeptide chain, including the spatial relationships between secondary structure elements. This structure is stabilized by a variety of interactions:
- Disulfide bonds: Covalent bonds between cysteine residues.
- Hydrogen bonds: Interactions between polar side chains.
- Ionic bonds (salt bridges): Electrostatic interactions between oppositely charged side chains.
- Hydrophobic interactions: Clustering of nonpolar side chains in the protein's interior, away from the surrounding water molecules.
The tertiary structure is crucial for protein function, as it creates a specific three-dimensional shape with active sites or binding pockets that are essential for interacting with other molecules.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains (subunits) assembled into a larger complex. This overall arrangement is the quaternary structure. Interactions between subunits are similar to those stabilizing tertiary structure: disulfide bonds, hydrogen bonds, ionic bonds, and hydrophobic interactions. Hemoglobin, for example, has a quaternary structure consisting of four subunits.
Protein Functions: A Diverse Array of Roles
Proteins perform a vast array of functions in living organisms. Their diverse roles are a direct consequence of their diverse structures:
- Enzymes: Catalyze biochemical reactions.
- Structural proteins: Provide structural support, like collagen in connective tissues.
- Transport proteins: Carry molecules across membranes, like hemoglobin carrying oxygen.
- Motor proteins: Generate movement, like myosin in muscle contraction.
- Hormones: Chemical messengers that regulate physiological processes, like insulin.
- Receptors: Bind to specific molecules, initiating cellular responses.
- Antibodies: Part of the immune system, recognizing and neutralizing foreign substances.
- Storage proteins: Store essential nutrients, like ferritin storing iron.
Factors Influencing Protein Structure and Function
Several factors can affect the structure and function of a protein:
- Temperature: High temperatures can denature proteins by disrupting weak interactions like hydrogen bonds.
- pH: Changes in pH can alter the charge of amino acid side chains, disrupting ionic interactions and protein structure.
- Salt concentration: High salt concentrations can disrupt ionic interactions.
- Reducing agents: Agents that break disulfide bonds can alter protein structure.
Protein Misfolding and Disease
When proteins misfold, they can lose their function and potentially become harmful. This misfolding is implicated in a variety of diseases, including Alzheimer's disease, Parkinson's disease, and cystic fibrosis. The accumulation of misfolded proteins can lead to aggregation and cellular dysfunction.
Conclusion: The Polymer of Amino Acids – A Marvel of Nature
In conclusion, a polymer of amino acids is a protein, a complex and versatile biomolecule essential for life. The linear sequence of amino acids, the primary structure, dictates the higher levels of protein structure – secondary, tertiary, and quaternary – which in turn determine the protein's function. Understanding the intricate relationships between amino acid sequence, protein structure, and protein function is crucial for unraveling the complexities of biological systems and for developing new treatments for protein-related diseases. The study of proteins continues to be a vibrant and rapidly evolving field, constantly revealing new insights into the fundamental processes of life.
Latest Posts
Latest Posts
-
Minerals Are Formed By The Process Of
Mar 17, 2025
-
M7 9 3 Perimeters And Areas Of Comp Fig
Mar 17, 2025
-
Investigation Mitosis And Cancer Answer Key
Mar 17, 2025
-
Where Is Halogens On The Periodic Table
Mar 17, 2025
-
Function Of A Stage On A Microscope
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is Polymer Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
