Where Is Halogens On The Periodic Table
Muz Play
Mar 17, 2025 · 6 min read
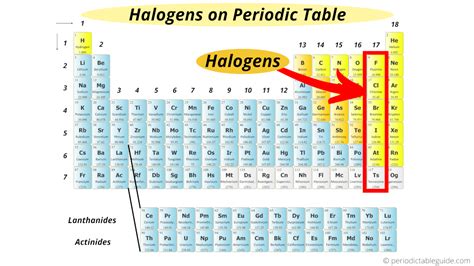
Table of Contents
Where Are the Halogens on the Periodic Table? A Deep Dive into Group 17
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its layout is crucial for grasping the behavior and characteristics of different elements. Within this organized system lies a fascinating group known as the halogens, a family of nonmetals with strikingly similar properties and a significant presence in our daily lives. But where exactly are the halogens on the periodic table? This comprehensive guide will not only answer that question but will also delve deep into their properties, reactivity, and applications.
Locating the Halogens: Group 17
The halogens are located in Group 17 (or VIIA) of the periodic table. This group is found on the right-hand side of the table, next to the noble gases (Group 18). You'll find them in the second to last column. Their positioning reflects their electron configuration and resulting chemical behavior.
Identifying the Halogen Family Members
The halogen family consists of five naturally occurring elements:
- Fluorine (F): Atomic number 9. A pale yellow, highly reactive gas.
- Chlorine (Cl): Atomic number 17. A greenish-yellow gas, also highly reactive.
- Bromine (Br): Atomic number 35. The only non-metallic liquid element at room temperature, a reddish-brown liquid.
- Iodine (I): Atomic number 53. A dark grey-black solid that sublimes (transitions directly from solid to gas) at room temperature, producing violet vapor.
- Astatine (At): Atomic number 85. A radioactive element with a very short half-life, making it extremely rare and difficult to study. Its properties are extrapolated based on its position in the periodic table.
Tennessine (Ts), with atomic number 117, is also considered a halogen, although its properties are still under investigation due to its synthetic nature and extremely short lifespan.
Understanding the Properties of Halogens: A Closer Look
The halogens share several key characteristics that stem directly from their similar electron configurations. These properties dictate their reactivity and applications.
Electron Configuration and Reactivity
The defining characteristic of halogens is their electron configuration. They all have seven valence electrons—electrons in their outermost shell. This means they are only one electron short of achieving a stable octet (eight valence electrons), a configuration that resembles the noble gases and imparts exceptional stability. This "one electron short" characteristic makes halogens highly reactive, aggressively seeking to gain that one electron through chemical bonding.
This high reactivity manifests in several ways:
- High electronegativity: Halogens have high electronegativity, meaning they strongly attract electrons in a chemical bond. This contributes to their ability to form stable compounds with a wide range of elements.
- Strong oxidizing agents: Their tendency to gain an electron makes them excellent oxidizing agents. They readily accept electrons from other substances, causing oxidation in the process.
- Formation of halide ions: When halogens react, they typically gain one electron to form negatively charged ions called halide ions (F⁻, Cl⁻, Br⁻, I⁻, At⁻). These ions are relatively stable and are found in numerous compounds.
Physical Properties: A Diverse Family
While their chemical properties are remarkably similar, their physical properties show a clear trend as you move down the group:
- State of matter: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid at room temperature. This change reflects increasing intermolecular forces as the atomic size increases.
- Color: The color of halogens deepens as you go down the group. Fluorine is pale yellow, chlorine is greenish-yellow, bromine is reddish-brown, and iodine is dark grey-black. Their vapor colors are also distinct, with iodine producing a characteristic violet vapor.
- Melting and boiling points: Melting and boiling points generally increase as you descend the group, reflecting the stronger intermolecular forces between larger halogen atoms.
- Atomic and ionic radius: Atomic and ionic radii increase down the group due to the addition of electron shells. This impacts reactivity; the larger atoms have less tightly held outer electrons, resulting in slightly lower reactivity.
Halogen Applications: From Everyday Life to Advanced Technology
Halogens and their compounds have a wide array of applications across diverse fields, reflecting their unique chemical properties.
Everyday Uses: Essential Components
Many halogen compounds are integral to our daily lives:
- Fluorine in toothpaste: Fluoride ions (from fluoride compounds) strengthen tooth enamel, preventing cavities. This is a crucial application for maintaining oral health.
- Chlorine in water treatment: Chlorine is a potent disinfectant, widely used to purify drinking water and swimming pools, preventing the spread of waterborne diseases.
- Iodine in salt: Iodized salt provides essential iodine, preventing iodine deficiency disorders. Iodine is critical for thyroid hormone production.
- Bromine in flame retardants: Certain bromine compounds are used as flame retardants in textiles and plastics, improving fire safety.
Industrial Applications: Driving Innovation
The industrial applications of halogens and their compounds are extensive:
- Chlorine in the production of PVC: Polyvinyl chloride (PVC) is a widely used plastic made using chlorine. It's utilized in pipes, flooring, and many other products.
- Fluorine in refrigerants: Certain fluorocarbons were once commonly used as refrigerants, but their contribution to ozone depletion led to their phasing out in favor of more environmentally friendly alternatives.
- Iodine in photography: Silver iodide is used in photographic film and plates, contributing to image formation.
- Halogens in organic synthesis: Halogens and their compounds are crucial reagents in organic chemistry, used in the synthesis of numerous organic molecules, including pharmaceuticals and agrochemicals.
Advanced Applications: Pushing Technological Boundaries
Halogens play a significant role in advanced technologies:
- Fluorine in semiconductors: Fluorine-containing compounds are used in the manufacturing of semiconductors, essential components of electronics.
- Chlorine in pharmaceuticals: Chlorine is incorporated into many pharmaceutical compounds, influencing their properties and biological activity.
- Halogens in lasers: Certain halogen compounds are used in lasers, generating specific wavelengths of light for various applications, including medical treatments and scientific research.
Environmental Concerns: Balancing Progress and Sustainability
While the applications of halogens are widespread and beneficial, some of their uses have raised environmental concerns:
- Ozone depletion: Certain chlorofluorocarbons (CFCs) and halons were found to deplete the ozone layer, which protects us from harmful ultraviolet radiation. International agreements like the Montreal Protocol led to the phasing out of these ozone-depleting substances.
- Pollution: The release of some halogen compounds into the environment can lead to pollution and pose risks to ecosystems and human health. Careful management and responsible disposal of halogen-containing waste are crucial.
The Future of Halogen Research and Applications
Research on halogens continues to evolve, focusing on:
- Developing environmentally friendly alternatives: The search for safer and more sustainable substitutes for ozone-depleting substances and other environmentally harmful halogen compounds is ongoing.
- Exploring new applications: Researchers are constantly exploring new applications for halogens and their compounds in various fields, including medicine, materials science, and energy technology.
- Understanding the behavior of astatine and tennessine: More research is needed to fully characterize the properties and behavior of the heavier halogens, astatine and tennessine, due to their radioactivity and limited availability.
In conclusion, understanding where the halogens are located on the periodic table is fundamental to appreciating their unique chemical behavior and diverse applications. From their crucial role in everyday products to their importance in advanced technologies, halogens have significantly impacted our lives. However, responsible use and environmental stewardship are essential to mitigate potential negative impacts and ensure the sustainable application of these remarkable elements.
Latest Posts
Latest Posts
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Where Is Halogens On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
