What Is Required For Osmosis To Occur
Muz Play
Mar 31, 2025 · 7 min read
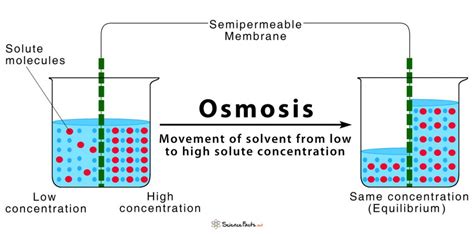
Table of Contents
What is Required for Osmosis to Occur? A Deep Dive into the Process
Osmosis, a fundamental process in biology and chemistry, plays a crucial role in various biological functions, from nutrient uptake in plants to maintaining cellular hydration in animals. Understanding what is required for osmosis to occur is key to comprehending its significance in living systems and various applications. This article will provide a comprehensive exploration of the necessary conditions, delving into the intricacies of semipermeable membranes, concentration gradients, and the driving force behind this vital process.
The Essentials: Semipermeable Membranes, Solutes, and Solvents
Osmosis, simply defined, is the net movement of water molecules across a selectively permeable membrane from a region of higher water concentration to a region of lower water concentration. This seemingly simple definition hides a complex interplay of several essential factors:
1. A Semipermeable Membrane: The Gatekeeper of Osmosis
The cornerstone of osmosis is the semipermeable membrane, also known as a selectively permeable membrane. This specialized membrane is crucial because it allows certain molecules, primarily water, to pass through while restricting the movement of others, namely solutes. This selective permeability is dictated by the membrane's structure and properties.
-
Membrane Structure: The composition of the membrane is key. Biological membranes, such as those found in cells, are primarily composed of a phospholipid bilayer. This bilayer has a hydrophobic (water-repelling) interior and hydrophilic (water-attracting) exterior, creating a selective barrier. The size and charge of molecules influence their ability to pass through. Small, uncharged molecules like water can typically pass through easily, while larger or charged molecules are impeded.
-
Aquaporins: Facilitated diffusion of water is greatly enhanced by the presence of aquaporins, specialized protein channels embedded within the membrane. These channels act as pores, allowing a significantly higher rate of water passage compared to simple diffusion across the lipid bilayer. The presence and density of aquaporins can significantly affect the rate of osmosis.
-
Artificial Membranes: Semipermeable membranes are not limited to biological systems. Artificial membranes are used extensively in various applications, including dialysis and water purification. These membranes are typically made from synthetic polymers designed to exhibit specific permeability characteristics.
2. A Concentration Gradient: The Driving Force
The second crucial requirement for osmosis is a concentration gradient. This refers to the difference in the concentration of water molecules (or, conversely, the concentration of solutes) across the membrane. Osmosis is driven by the tendency of the system to reach equilibrium—a state where the concentration of water is equal on both sides of the membrane.
-
Water Potential: The concept of water potential is often used to describe the tendency of water to move from one area to another. Water potential is influenced by several factors, including solute concentration, pressure, and gravity. Water moves from areas of high water potential to areas of low water potential. A higher solute concentration leads to lower water potential.
-
Solute Concentration: The concentration of solutes dissolved in the water is directly related to the water concentration. A solution with a high solute concentration has a lower water concentration and vice versa. This difference in concentration drives the movement of water.
-
Establishing the Gradient: The concentration gradient can be established naturally or artificially. In biological systems, differences in solute concentration arise due to metabolic processes and selective transport across membranes. In laboratory settings, researchers can create controlled gradients by using different solutions on either side of a semipermeable membrane.
3. Water as the Solvent: The Moving Molecule
Water is the universal solvent in biological systems and is the primary molecule involved in osmosis. The polar nature of water molecules allows them to interact with other polar molecules and ions, facilitating their dissolution. In osmosis, it's the movement of water molecules, not the solutes, that directly causes the net flow across the membrane.
-
Hydrogen Bonding: Water molecules form hydrogen bonds with each other and with other polar molecules. These bonds contribute to the cohesive and adhesive properties of water, influencing its movement across membranes.
-
Water as a Medium: Water acts not just as the moving substance but also as the medium in which the solutes are dissolved. The properties of water, like its polarity and high dielectric constant, affect the behavior of the solutes and their interaction with the membrane.
Understanding Osmosis: Hypotonic, Isotonic, and Hypertonic Solutions
The direction and extent of water movement during osmosis are largely determined by the tonicity of the solutions on either side of the membrane. Tonicity describes the relative concentration of solutes in two solutions separated by a selectively permeable membrane.
1. Hypotonic Solution: Water Flows In
A hypotonic solution has a lower solute concentration compared to the solution on the other side of the membrane. This means it has a higher water concentration. Consequently, water moves from the hypotonic solution (high water potential) into the other solution (low water potential) across the semipermeable membrane. This influx of water can cause cells to swell and potentially burst (lyse) in the case of animal cells. Plant cells, however, are protected by their rigid cell walls, which prevent lysis and result in turgor pressure.
2. Isotonic Solution: No Net Water Movement
An isotonic solution has an equal concentration of solutes compared to the solution on the other side of the membrane. In this case, there is no net movement of water across the membrane. Water molecules still move back and forth, but the movement is equal in both directions, resulting in no overall change in volume. This is generally considered the ideal environment for animal cells.
3. Hypertonic Solution: Water Flows Out
A hypertonic solution has a higher solute concentration compared to the solution on the other side of the membrane. This means it has a lower water concentration. Water moves from the solution with the lower solute concentration (higher water potential) into the hypertonic solution (lower water potential). This efflux of water causes cells to shrink or shrivel (crenate) in the case of animal cells. In plant cells, this causes plasmolysis, where the cell membrane pulls away from the cell wall.
Applications and Importance of Osmosis
Osmosis is not merely a theoretical concept; it plays a vital role in numerous biological processes and has widespread applications in various fields:
-
Plant Physiology: Osmosis is crucial for water uptake by plant roots and maintaining turgor pressure, which keeps plants upright and supports their growth.
-
Animal Physiology: Osmosis plays a critical role in maintaining the proper hydration and electrolyte balance in animal cells and tissues. The kidneys utilize osmosis to regulate water and solute levels in the blood.
-
Water Purification: Reverse osmosis, a process that uses pressure to force water across a semipermeable membrane against its concentration gradient, is used to purify water by removing impurities like salts and minerals.
-
Dialysis: Dialysis machines use semipermeable membranes to remove waste products from the blood of patients with kidney failure. This process relies on the principle of osmosis and diffusion.
-
Food Preservation: Osmosis is used in food preservation techniques like pickling and canning. High concentrations of salt or sugar draw water out of microorganisms, inhibiting their growth and extending the shelf life of the food.
Factors Affecting the Rate of Osmosis
The rate of osmosis is not constant; several factors influence its speed:
-
Temperature: Higher temperatures generally increase the rate of osmosis as they increase the kinetic energy of water molecules.
-
Concentration Gradient: A steeper concentration gradient (larger difference in water potential) leads to a faster rate of osmosis.
-
Membrane Permeability: The permeability of the membrane greatly affects the rate. Membranes with more aquaporins or higher permeability to water will allow faster osmosis.
-
Surface Area: A larger surface area of the membrane increases the rate of osmosis as more water molecules can cross simultaneously.
-
Distance: The distance the water molecules have to travel across the membrane also influences the rate. Thinner membranes facilitate faster osmosis.
Conclusion: A Vital Process with Far-Reaching Consequences
Osmosis is a fundamental biological process with significant implications for living organisms and various technological applications. The interplay between semipermeable membranes, concentration gradients, and the properties of water drives this crucial process. Understanding the requirements for osmosis is vital for grasping its role in maintaining cellular homeostasis, regulating water balance, and influencing numerous other physiological and technological processes. From the smallest cell to large-scale water purification systems, osmosis continues to be a central concept in biology, chemistry, and engineering.
Latest Posts
Latest Posts
-
Competing Visions A History Of California
Apr 01, 2025
-
What Are Three Main Ideas Of The Cell Theory
Apr 01, 2025
-
Identify All Correct Statements About The Basic Function Of Fermentation
Apr 01, 2025
-
Different Blocks On The Periodic Table
Apr 01, 2025
-
How Can You Determine The Optimum Ph Of An Enzyme
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is Required For Osmosis To Occur . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
