Different Blocks On The Periodic Table
Muz Play
Apr 01, 2025 · 7 min read
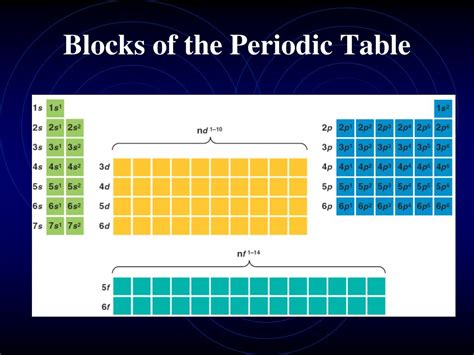
Table of Contents
Delving Deep into the Periodic Table: Exploring Different Blocks of Elements
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. While seemingly a simple grid, it reveals intricate patterns and relationships between elements, categorized into distinct blocks: s-block, p-block, d-block, and f-block. Understanding these blocks is crucial to comprehending the diverse properties and behaviors of elements. This comprehensive guide explores each block in detail, examining their characteristics, key elements, and applications.
The s-Block: Alkali and Alkaline Earth Metals
The s-block, located on the far left of the periodic table, comprises Groups 1 and 2: the alkali metals (Group 1) and the alkaline earth metals (Group 2). These elements are characterized by their valence electrons residing in the s subshell. This shared electronic configuration dictates their chemical behavior, resulting in similar properties within each group.
Alkali Metals (Group 1): Reactivity and Uniqueness
Alkali metals, from lithium (Li) to francium (Fr), are highly reactive due to their single valence electron. This electron is easily lost, forming +1 ions. Their reactivity increases significantly as you descend the group, with cesium and francium being exceptionally reactive. This high reactivity necessitates their storage under oil or inert atmospheres to prevent reactions with air and moisture.
Key characteristics of alkali metals:
- Low ionization energies: Easily lose their valence electron.
- Low electronegativity: Tend to lose electrons rather than gain them.
- Soft and silvery-white: Exhibit metallic properties.
- Low melting and boiling points: Relatively low compared to other metals.
- Form ionic compounds: Readily react with nonmetals to form ionic compounds.
Applications of alkali metals:
- Lithium: Used in batteries, ceramics, and lubricants. Lithium-ion batteries power many portable electronic devices.
- Sodium: Essential component of table salt (NaCl), used in streetlights (sodium vapor lamps), and in the production of other chemicals.
- Potassium: Crucial for plant growth and plays vital roles in human physiology.
- Rubidium and Cesium: Employed in atomic clocks and specialized optical applications.
Alkaline Earth Metals (Group 2): Building Blocks and Beyond
Alkaline earth metals, from beryllium (Be) to radium (Ra), possess two valence electrons in their s subshell. These elements are less reactive than alkali metals but still exhibit significant reactivity, particularly with oxygen and water. They form +2 ions readily.
Key characteristics of alkaline earth metals:
- Higher ionization energies than alkali metals: Losing two electrons requires more energy.
- Higher melting and boiling points than alkali metals: Stronger metallic bonding.
- Relatively harder and denser than alkali metals: Stronger metallic bonds.
- Form ionic compounds: Similar to alkali metals but with a +2 charge.
Applications of alkaline earth metals:
- Beryllium: Used in aerospace alloys due to its high strength-to-weight ratio.
- Magnesium: Lightweight metal used in alloys for aircraft and automobiles. Also used in flash photography and flares.
- Calcium: Essential for bone structure and numerous biological processes.
- Strontium: Used in fireworks for its red color.
- Barium: Used in medical imaging (barium sulfate) and in some specialized ceramics.
The p-Block: A Diverse Range of Properties
The p-block, occupying a larger portion of the periodic table, includes elements from Groups 13 to 18. These elements have their valence electrons in the p subshell, leading to a vast diversity of properties. This block encompasses metals, metalloids (semi-metals), and nonmetals.
Group 13: Boron Family
The boron family, starting with boron (B), exhibits a gradual transition from nonmetallic to metallic behavior as you descend the group. Boron is a metalloid, while aluminum (Al), gallium (Ga), indium (In), and thallium (Tl) are metals. They have three valence electrons.
Key characteristics and applications:
- Boron: Used in borax (a cleaning agent) and borosilicate glass (Pyrex).
- Aluminum: Lightweight, corrosion-resistant metal widely used in packaging, construction, and transportation.
- Gallium: Used in semiconductors and LEDs.
- Indium: Used in LCD screens and solar cells.
- Thallium: Highly toxic and has limited applications.
Group 14: Carbon Family
The carbon family, starting with carbon (C), displays a striking range of properties. Carbon exists in various allotropes (diamond, graphite, fullerenes), demonstrating its versatility. Silicon (Si) and germanium (Ge) are metalloids, while tin (Sn) and lead (Pb) are metals.
Key characteristics and applications:
- Carbon: Forms the basis of organic chemistry and is essential for life. Used in steelmaking, graphite pencils, and diamonds.
- Silicon: The primary component of computer chips and semiconductors. Also used in solar cells.
- Germanium: Used in semiconductors and fiber optics.
- Tin: Used in coatings (tin cans) and alloys.
- Lead: Toxic and its use is increasingly restricted. Historically used in batteries and paints.
Group 15: Nitrogen Family
The nitrogen family, including nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi), shows a trend towards increasing metallic character down the group. Nitrogen and phosphorus are nonmetals, arsenic and antimony are metalloids, and bismuth is a metal.
Key characteristics and applications:
- Nitrogen: Major component of the atmosphere, crucial for life, and used in fertilizers.
- Phosphorus: Essential for fertilizers and detergents. Exists in white and red allotropic forms.
- Arsenic: Toxic and has limited applications.
- Antimony: Used in flame retardants and alloys.
- Bismuth: Used in pharmaceuticals and low-melting alloys.
Group 16: Oxygen Family (Chalcogens)
The oxygen family includes oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po). Oxygen is a nonmetal, sulfur is a nonmetal, selenium and tellurium are metalloids, and polonium is a radioactive metal.
Key characteristics and applications:
- Oxygen: Essential for respiration and combustion.
- Sulfur: Used in sulfuric acid production, vulcanization of rubber, and fertilizers.
- Selenium: Used in photocopiers and solar cells.
- Tellurium: Used in some alloys and semiconductors.
- Polonium: Radioactive and has limited applications.
Group 17: Halogens
Halogens (Group 17), including fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At), are highly reactive nonmetals. They readily gain an electron to form -1 ions. Their reactivity decreases down the group.
Key characteristics and applications:
- Fluorine: Used in fluorinated compounds (Teflon) and toothpastes.
- Chlorine: Used in water purification, disinfectants, and PVC production.
- Bromine: Used in flame retardants and photographic chemicals.
- Iodine: Essential nutrient used in disinfectants and thyroid hormone production.
- Astatine: Radioactive and has limited applications.
Group 18: Noble Gases
Noble gases (Group 18), including helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), are inert gases with full valence electron shells. They are very unreactive.
Key characteristics and applications:
- Helium: Used in balloons, cryogenics, and MRI machines.
- Neon: Used in neon signs.
- Argon: Used in welding and as an inert atmosphere.
- Krypton: Used in some lighting applications.
- Xenon: Used in some lighting applications and anesthesia.
- Radon: Radioactive and a health hazard.
The d-Block: Transition Metals
The d-block, located in the center of the periodic table, comprises the transition metals. These elements have their valence electrons in the d subshell, exhibiting variable oxidation states and often forming colored compounds. They are generally good conductors of heat and electricity.
Key characteristics of transition metals:
- Variable oxidation states: Can lose different numbers of electrons, leading to diverse compounds.
- Formation of colored compounds: Due to the d-electron transitions.
- Catalytic activity: Many transition metals act as catalysts in chemical reactions.
- Formation of complexes: Transition metals form complex ions with ligands.
- High melting and boiling points: Strong metallic bonding.
Applications of transition metals:
Numerous applications exist for transition metals, including iron (steel production), copper (electrical wiring), nickel (alloys), platinum (catalysts), and many others. Their properties make them essential in various industries.
The f-Block: Lanthanides and Actinides
The f-block, situated at the bottom of the periodic table, comprises the lanthanides (rare earth elements) and actinides. These elements have their valence electrons in the f subshell. Actinides are all radioactive.
Key characteristics of f-block elements:
- Similar chemical properties: Lanthanides exhibit similar chemical properties due to the shielding effect of the f electrons.
- Radioactivity: Actinides are all radioactive, with varying degrees of radioactivity.
- Applications in specialized technologies: Lanthanides are crucial in various technologies, such as magnets, lasers, and lighting. Actinides are used in nuclear technology.
This detailed exploration of the different blocks within the periodic table highlights the diverse properties and applications of elements based on their atomic structure. From the highly reactive alkali metals to the inert noble gases and the versatile transition metals, each block contributes significantly to the rich tapestry of chemical behavior and technological advancements. Understanding the periodic table's organization is a fundamental step in comprehending the world around us.
Latest Posts
Latest Posts
-
How To Add Radical Expressions With Variables
Apr 02, 2025
-
Give The Iupac Name Of The Carboxylic Acid Below
Apr 02, 2025
-
What Is An Abbreviated Electron Configuration
Apr 02, 2025
-
Difference Between A Strong Base And A Weak Base
Apr 02, 2025
-
Use The Graph To Find The Indicated Function Values
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Different Blocks On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
