What Is The Basic Unit Of Measurement
Muz Play
Mar 31, 2025 · 6 min read
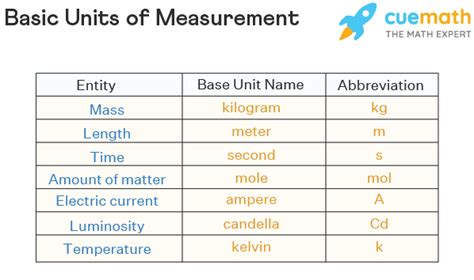
Table of Contents
What is the Basic Unit of Measurement? A Deep Dive into the International System of Units (SI)
The question, "What is the basic unit of measurement?" isn't as simple as it sounds. It depends heavily on what you're measuring and the system of measurement you're using. While there's no single "basic" unit applicable to everything, the International System of Units (SI), often called the metric system, provides a foundational framework for consistent and universally understood measurements. This article will delve deep into the SI system, exploring its seven base units, derived units, prefixes, and their significance in various scientific and everyday applications. We’ll also briefly touch upon other historical and less common systems.
The Seven Base Units of the SI System
The SI system's strength lies in its coherence. It builds upon seven fundamental units, defining all other units through mathematical relationships. These seven base units are:
1. Meter (m): The Unit of Length
The meter, symbolized by 'm', is the base unit of length. Originally defined in relation to the Earth's circumference, its definition has evolved over time. Currently, it's defined using the speed of light in a vacuum. A meter is the distance light travels in a vacuum in 1/299,792,458 of a second. This incredibly precise definition ensures consistent measurements worldwide, irrespective of location or experimental setup. Understanding the meter is crucial in fields like surveying, construction, astronomy, and countless other disciplines.
2. Kilogram (kg): The Unit of Mass
The kilogram, denoted by 'kg', is the base unit of mass. Unlike the meter, the kilogram's definition is still based on a physical artifact—the International Prototype Kilogram (IPK)—a platinum-iridium cylinder kept under highly controlled conditions in Sèvres, France. However, efforts are underway to redefine the kilogram based on fundamental physical constants, similar to the meter, ensuring greater stability and accessibility. Mass is a measure of the amount of matter in an object and is distinct from weight, which is the force of gravity on that mass. The kilogram is fundamental in physics, chemistry, engineering, and everyday life, affecting everything from grocery shopping to industrial production.
3. Second (s): The Unit of Time
The second, represented by 's', is the base unit of time. It's defined using the cesium-133 atom's radiation frequency. Specifically, one second is defined as the duration of 9,192,631,770 periods of the radiation corresponding to the transition between two hyperfine levels of the ground state of the cesium-133 atom. This atomic clock-based definition guarantees unparalleled accuracy and consistency, critical for technologies like GPS navigation, telecommunications, and scientific experimentation.
4. Ampere (A): The Unit of Electric Current
The ampere, symbolized by 'A', is the base unit of electric current. It's defined using the force between two infinitely long, parallel conductors carrying a constant current. One ampere is the constant current that, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed one meter apart in vacuum, would produce between these conductors a force equal to 2 x 10⁻⁷ newton per meter of length. This definition ensures a robust and reproducible measurement of electrical current, crucial for electrical engineering, electronics, and numerous other fields.
5. Kelvin (K): The Unit of Thermodynamic Temperature
The Kelvin, represented by 'K', is the base unit of thermodynamic temperature. It's defined using the triple point of water, which is the temperature and pressure at which water coexists as solid, liquid, and gas. Zero Kelvin (0 K) represents absolute zero, the theoretical lowest possible temperature. Thermodynamic temperature is a fundamental concept in thermodynamics, physics, and chemistry, impacting fields ranging from materials science to climate modeling.
6. Mole (mol): The Unit of Amount of Substance
The mole, symbolized by 'mol', is the base unit of the amount of substance. One mole contains exactly 6.02214076 × 10²³ elementary entities. This number is known as Avogadro's number, and it represents the number of atoms, molecules, ions, or other specified entities in one mole of a substance. The mole is crucial in chemistry and related fields, enabling precise measurements and calculations involving chemical reactions and stoichiometry.
7. Candela (cd): The Unit of Luminous Intensity
The candela, symbolized by 'cd', is the base unit of luminous intensity. It's defined in terms of the radiant intensity of monochromatic light of frequency 540 × 10¹² hertz, a frequency that corresponds to green light, which the human eye is most sensitive to. One candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 × 10¹² hertz and that has a radiant intensity in that direction of 1/683 watt per steradian. The candela is critical for lighting design, photography, and applications involving human perception of light.
Derived Units: Building Blocks of Measurement
The seven base units form the foundation upon which all other units in the SI system are built. These are called derived units. They are obtained by combining base units using mathematical operations like multiplication and division. Examples include:
- Square meter (m²): Area
- Cubic meter (m³): Volume
- Meter per second (m/s): Speed
- Newton (N = kg·m/s²): Force
- Pascal (Pa = N/m²): Pressure
- Joule (J = kg·m²/s²): Energy
- Watt (W = J/s): Power
- Volt (V = kg·m²/s³·A⁻¹): Electric potential
Prefixes: Expanding the Range of Measurement
The SI system utilizes prefixes to extend the range of each base unit, allowing for the convenient expression of extremely large or small quantities. These prefixes are based on powers of 10, making conversions straightforward. Some commonly used prefixes include:
- kilo (k): 10³ (1000)
- mega (M): 10⁶ (1,000,000)
- giga (G): 10⁹ (1,000,000,000)
- tera (T): 10¹² (1,000,000,000,000)
- milli (m): 10⁻³ (0.001)
- micro (µ): 10⁻⁶ (0.000001)
- nano (n): 10⁻⁹ (0.000000001)
- pico (p): 10⁻¹² (0.000000000001)
Other Systems of Measurement
While the SI system is the dominant global standard, other systems of measurement exist, albeit with less widespread use. The most notable is the Imperial system, still prevalent in some countries, including the United States. The Imperial system uses units like feet, pounds, and gallons, which are often less convenient for scientific calculations and international collaboration due to their complex relationships and lack of coherence compared to the SI system.
The Importance of Consistent Measurement
The use of a unified system of measurement like the SI is crucial for several reasons:
- Scientific Collaboration: It facilitates clear communication and reproducibility of scientific results across geographical boundaries.
- International Trade: It streamlines commerce and reduces ambiguities in product specifications.
- Technological Advancement: A consistent system is vital for technological progress, particularly in fields like engineering and manufacturing.
- Everyday Life: Standardized units simplify everyday tasks, from cooking to construction.
Conclusion: The Foundation of Understanding
The basic unit of measurement, while not a single entity, is fundamentally defined by the SI system's seven base units. These base units, along with derived units and prefixes, offer a comprehensive and universally accepted framework for quantifying the physical world. Understanding this system is crucial for anyone involved in science, technology, engineering, medicine, or indeed any field where precise measurement is essential. The continuous refinement and evolution of the SI system, particularly in redefining units based on fundamental physical constants, underscore its commitment to accuracy, consistency, and future-proofing our understanding of the universe. From the smallest subatomic particles to the vast expanse of the cosmos, the SI system serves as a universal language of measurement, enabling us to explore, understand, and interact with the world around us in a more precise and effective way.
Latest Posts
Latest Posts
-
What Are The Different Types Of Fossils
Apr 01, 2025
-
Compare And Contrast Monotheism Polytheism And Animism
Apr 01, 2025
-
Is The Five Carbon Sugar Found In Dna
Apr 01, 2025
-
Builds A New Dna Strand By Adding Complementary Bases
Apr 01, 2025
-
Which Of The Following Situations Will Lead To Natural Selection
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Basic Unit Of Measurement . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
