What Is The Concentration Of Water
Muz Play
Apr 01, 2025 · 7 min read
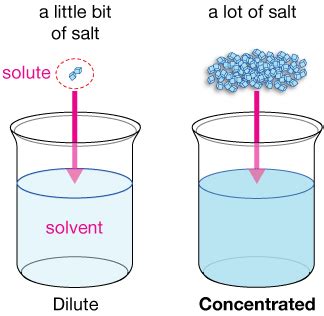
Table of Contents
What is the Concentration of Water? Understanding Molarity, Molality, and More
Water, the elixir of life, is ubiquitous on our planet. But beyond its simple chemical formula (H₂O), understanding the concentration of water opens a fascinating window into chemistry, biology, and various scientific disciplines. The concentration of water isn't a single, fixed number, but rather a variable that depends on how we define and measure it. This article will explore the different ways to express water's concentration, delving into molarity, molality, and other relevant concepts.
Defining Concentration
Before we dive into the specifics of water concentration, let's establish a fundamental understanding of what "concentration" means in chemistry. Concentration describes the amount of a solute dissolved in a given amount of solvent or solution. A solute is the substance being dissolved (in this case, often a substance dissolved in water), and the solvent is the substance doing the dissolving (usually water itself). The solution is the homogenous mixture resulting from the solute and solvent. Higher concentrations mean more solute per unit volume or mass of the solution, while lower concentrations indicate less solute.
Expressing Water Concentration: A Multifaceted Approach
The concentration of water can be expressed in several ways, depending on the context and the desired level of precision. Here are some common methods:
1. Molarity (M)
Molarity is perhaps the most frequently used unit for expressing concentration in chemistry. It represents the number of moles of solute per liter of solution. The formula is:
Molarity (M) = moles of solute / liters of solution
In the context of water, it’s important to remember that we are usually referring to the concentration of a solute dissolved in water, not the concentration of water itself. Pure water, being the solvent, has a molarity that depends on its density and molar mass. At 25°C, the density of water is approximately 1 gram per milliliter (g/mL), and its molar mass is approximately 18 g/mol. Therefore, the molarity of pure water at this temperature can be calculated as follows:
- Convert density to moles per liter: 1 g/mL * (1000 mL/1 L) = 1000 g/L
- Convert grams to moles: 1000 g/L / 18 g/mol ≈ 55.56 mol/L
Therefore, the molarity of pure water at 25°C is approximately 55.56 M. This exceptionally high concentration reflects the fact that water is the solvent in most aqueous solutions.
2. Molality (m)
Unlike molarity, molality (m) is defined as the number of moles of solute per kilogram of solvent. The formula is:
Molality (m) = moles of solute / kilograms of solvent
Molality is less sensitive to temperature changes than molarity because it's based on mass rather than volume. This makes it a more suitable choice for precise measurements in situations where temperature fluctuations might significantly affect volume. Again, when discussing water’s concentration within a solution, we are referring to the molality of the solute, not water itself. The molality of water as a solvent would be undefined in the typical application of this concept.
3. Mole Fraction (χ)
The mole fraction of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution. The formula is:
Mole fraction (χ) = moles of component / total moles of all components
For a binary solution (like a solute dissolved in water), the mole fraction of water (χ<sub>water</sub>) would be:
χ<sub>water</sub> = moles of water / (moles of water + moles of solute)
In a dilute solution, the mole fraction of water is close to 1, while in concentrated solutions, it approaches 0. It's crucial to note that this method helps express the relative amount of water compared to other components.
4. Mass Percentage (%)
Mass percentage (or weight percentage) expresses the concentration of a component as the mass of that component divided by the total mass of the solution, multiplied by 100%. The formula is:
Mass percentage (%) = (mass of component / total mass of solution) * 100%
This is a straightforward method and is often used for practical purposes, particularly in cases where precise molar masses may not be readily available.
5. Volume Percentage (%)
Similar to mass percentage, volume percentage represents the volume of a component divided by the total volume of the solution, multiplied by 100%. The formula is:
Volume percentage (%) = (volume of component / total volume of solution) * 100%
This method is frequently used when dealing with liquids, but it's essential to remember that volumes aren't always additive. Mixing two liquids might not result in a total volume that's simply the sum of the individual volumes.
6. Parts Per Million (ppm) and Parts Per Billion (ppb)
These units are frequently used to express very low concentrations of solutes in a solution. They represent the number of parts of solute per million or billion parts of solution. They are often used for expressing trace amounts of contaminants or impurities in water. They can be expressed by mass or volume:
- ppm (mass) = (mass of solute / mass of solution) * 10<sup>6</sup>
- ppb (mass) = (mass of solute / mass of solution) * 10<sup>9</sup>
Water Concentration in Different Contexts
The choice of how to express water concentration depends heavily on the specific application:
-
Biological Systems: In biology, the concentration of water plays a vital role in cellular processes, osmosis, and maintaining the proper physiological environment for organisms. Often, mole fractions or osmotic pressure (a related concept that depends on concentration) are used to describe the water's contribution to a cell's environment.
-
Chemistry: Chemists use molarity, molality, and mole fractions frequently when performing experiments and calculations involving solutions. Knowing the precise concentration of water in a reaction mixture is critical for accurate results.
-
Environmental Science: Environmental scientists often use ppm and ppb to quantify pollutants and contaminants present in water sources. This helps assess water quality and potential environmental risks.
-
Food Science: Water activity (a related concept based on the partial vapor pressure of water) is a crucial factor in food preservation and shelf life. It measures the availability of water for microbial growth and chemical reactions.
-
Industrial Processes: In many industrial processes that use water as a solvent or reactant, the precise concentration of water needs to be carefully controlled to ensure product quality and efficient operation.
Practical Applications and Considerations
The concept of water concentration extends beyond simple laboratory measurements. It has significant implications across many aspects of our lives:
-
Desalination: Desalination processes aim to increase the concentration of potable water by removing salts and minerals. Understanding the initial and final water concentration is essential for optimizing these techniques.
-
Irrigation: The water concentration in irrigation systems affects the growth and health of plants. The presence of salts or other dissolved substances can negatively impact plant development.
-
Pharmaceuticals: The concentration of water is critical in pharmaceutical preparations. Accurate concentration is vital for drug efficacy and safety.
-
Meteorology: The concentration of water vapor in the atmosphere plays a crucial role in weather patterns, cloud formation, and precipitation.
Conclusion: A Deeper Dive into the Essence of Water
Understanding water concentration is essential for a wide range of scientific, industrial, and environmental applications. While the molarity of pure water might seem like a simple calculation, the various ways to express water's concentration – from molarity and molality to mole fractions and ppm – highlight its multifaceted role in various systems. By grasping these concepts and their implications, we gain a more profound appreciation of this remarkable substance and its significance in our world. This knowledge forms a foundation for more advanced studies in fields like solution chemistry, biochemistry, and environmental science. Further exploration into topics like colligative properties (properties of solutions that depend on the concentration of solute particles, not their identity) and osmotic pressure will deepen one’s understanding of water's behavior in different systems.
Latest Posts
Latest Posts
-
Base Excision Repair Vs Mismatch Repair
Apr 02, 2025
-
Is Argon Metal Nonmetal Or Metalloid
Apr 02, 2025
-
What Is A Limiting Amino Acid In A Protein
Apr 02, 2025
-
Under What Conditions Are Gases Most Likely To Behave Ideally
Apr 02, 2025
-
How To Find The Vertical Asymptote Of A Limit
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Concentration Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
