What Is The Conjugate Base Of Hcl
Muz Play
Mar 18, 2025 · 6 min read
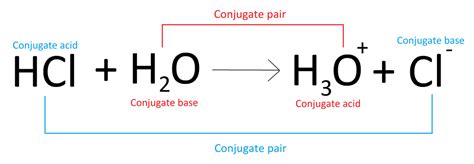
Table of Contents
- What Is The Conjugate Base Of Hcl
- Table of Contents
- What is the Conjugate Base of HCl? A Deep Dive into Acid-Base Chemistry
- Understanding Conjugate Acid-Base Pairs
- HCl: A Strong Acid
- Identifying the Conjugate Base of HCl
- Properties of the Chloride Ion (Cl⁻)
- Comparing HCl and Cl⁻: A Detailed Analysis
- The Importance of Conjugate Acid-Base Pairs in Equilibrium
- Applications of HCl and Cl⁻
- Further Exploration: Other Strong Acids and Their Conjugate Bases
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What is the Conjugate Base of HCl? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on hydrochloric acid (HCl) and its conjugate base. We will explore the definition of conjugate bases, the properties of HCl and its conjugate base, and the implications of this relationship in various chemical reactions and applications. We'll also touch upon related concepts to provide a comprehensive understanding.
Understanding Conjugate Acid-Base Pairs
According to Brønsted-Lowry acid-base theory, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. This relationship is crucial in understanding acid-base reactions and equilibrium. The conjugate base is essentially what remains of the acid after it has donated its proton.
Key Characteristics of Conjugate Acid-Base Pairs:
- They differ by only one proton (H⁺).
- They are related through a reversible reaction (an equilibrium).
- The strength of an acid is inversely related to the strength of its conjugate base (strong acids have weak conjugate bases, and vice versa).
HCl: A Strong Acid
Hydrochloric acid (HCl), also known as muriatic acid, is a strong acid. This means that it almost completely dissociates in aqueous solution, meaning it readily donates its proton. The dissociation reaction can be represented as:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
In this reaction, HCl acts as the acid, donating a proton (H⁺) to form the hydronium ion (H₃O⁺) in water (although we often simplify this to just H⁺ for convenience).
Identifying the Conjugate Base of HCl
Now, let's identify the conjugate base. Looking at the dissociation reaction above, when HCl donates its proton, what's left? The answer is the chloride ion (Cl⁻). Therefore, Cl⁻ is the conjugate base of HCl.
Properties of the Chloride Ion (Cl⁻)
The chloride ion, Cl⁻, is a relatively large and stable anion. This stability is due to the relatively high electronegativity of chlorine, which means it can effectively handle the extra electron. This stability is a key factor in the weakness of its conjugate acid HCl. The following summarizes its key properties:
- Weak conjugate base: Because HCl is a strong acid, Cl⁻ is a very weak conjugate base. This means it has a negligible tendency to accept a proton and reform HCl. In aqueous solutions, it barely reacts with water to reform HCl.
- Solubility: Chloride ions are highly soluble in water. This makes them readily available in many aqueous solutions.
- Reactivity: Chloride ions are relatively unreactive in most circumstances. Their lack of reactivity helps to ensure the stability of solutions containing them.
- Presence in biological systems: Chloride ions are crucial in various biological processes, playing a significant role in maintaining the balance of electrolytes in the body.
Comparing HCl and Cl⁻: A Detailed Analysis
| Feature | HCl (Hydrochloric Acid) | Cl⁻ (Chloride Ion) |
|---|---|---|
| Nature | Strong acid | Weak conjugate base |
| Proton donor/acceptor | Proton donor | Proton acceptor (but weakly) |
| Dissociation | Almost complete dissociation in water | Minimal tendency to react with water to form HCl |
| Reactivity | Highly reactive (corrosive) | Relatively unreactive |
| Solubility | Highly soluble in water | Highly soluble in water |
| pH | Very low (highly acidic) | Neutral or slightly basic (if in a solution of a strong base) |
The Importance of Conjugate Acid-Base Pairs in Equilibrium
The equilibrium between an acid and its conjugate base is described by the acid dissociation constant (Ka). For a general acid, HA, the equilibrium is:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The Ka is given by:
Ka = [H⁺][A⁻] / [HA]
For a strong acid like HCl, the Ka value is very large, indicating that the equilibrium heavily favors the products (H⁺ and Cl⁻). For weak acids, the Ka is much smaller. The pKa (the negative logarithm of Ka) is a more commonly used value, with lower pKa values indicating stronger acids.
Understanding the Ka value and pKa helps predict the behaviour of the acid-base system under various conditions. For instance, if we add a strong base to a solution of HCl, the hydroxide ions (OH⁻) from the base will react with the H⁺ ions, shifting the equilibrium to the left, consuming HCl and forming water. This demonstrates the interdependency of the acid and its conjugate base in maintaining equilibrium.
Applications of HCl and Cl⁻
Both HCl and Cl⁻ have numerous applications across various fields:
HCl:
- Industrial Processes: HCl is widely used in the production of various chemicals, including PVC (polyvinyl chloride) and other polymers.
- Food Production: It's used in the production of certain food products as an acidity regulator.
- Metal Cleaning: HCl is employed to clean metals before processes such as plating or welding.
- pH Control: HCl is utilized to adjust the pH of various solutions in industrial settings.
- Digestion: In the human body, hydrochloric acid in the stomach plays a crucial role in digestion.
Cl⁻:
- Electrolytes: Chloride ions are essential electrolytes in biological systems, contributing to maintaining fluid balance and nerve impulse transmission.
- Salt Production: The most common compound containing Cl⁻ is sodium chloride (NaCl), also known as common table salt.
- Industrial Processes: Chloride ions are involved in many industrial chemical processes.
- Medicine: Chloride salts have various medicinal applications.
Further Exploration: Other Strong Acids and Their Conjugate Bases
While HCl is a prime example, let's briefly consider other strong acids and their conjugate bases:
- Nitric acid (HNO₃): The conjugate base is the nitrate ion (NO₃⁻), which is a stable and relatively unreactive anion.
- Sulfuric acid (H₂SO₄): Sulfuric acid is a diprotic acid, meaning it can donate two protons. Its conjugate bases are the bisulfate ion (HSO₄⁻) and the sulfate ion (SO₄²⁻).
- Perchloric acid (HClO₄): Its conjugate base is the perchlorate ion (ClO₄⁻), also a stable and unreactive anion.
These examples further illustrate the principle that strong acids have weak conjugate bases due to their low propensity to accept a proton.
Conclusion
Understanding the conjugate base of an acid, particularly a strong acid like HCl, is essential for grasping fundamental concepts in acid-base chemistry and its various applications. The chloride ion (Cl⁻), the conjugate base of HCl, exhibits properties contrasting with its parent acid, primarily its weak basicity and high stability. This knowledge is crucial in predicting the behaviour of acid-base reactions and analyzing various chemical systems in industrial processes, biological functions, and many other aspects of chemistry. This article provides a strong foundation for exploring more complex acid-base concepts and reactions. Remember to always prioritize safety when handling acids and chemicals.
Latest Posts
Latest Posts
-
Is Main A Keyword In Fortran
Mar 18, 2025
-
How To Find Bond Dissociation Energy
Mar 18, 2025
-
Where Does Mrna Go After It Leaves The Nucleus
Mar 18, 2025
-
What Is The Difference Between Primary And Secondary Growth
Mar 18, 2025
-
Work Done By An Electric Field
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Base Of Hcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
