What Is The Difference Between A Reactant And A Product
Muz Play
Mar 26, 2025 · 6 min read
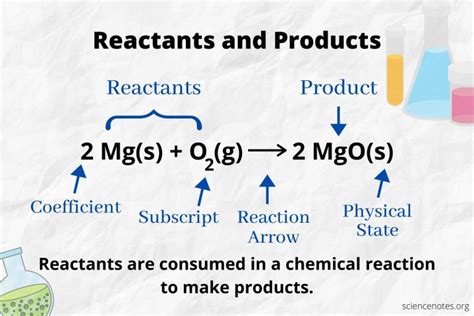
Table of Contents
What's the Difference Between a Reactant and a Product? A Comprehensive Guide
Understanding the difference between reactants and products is fundamental to grasping the core concepts of chemistry. While seemingly simple, a thorough understanding goes beyond a basic definition and delves into the intricacies of chemical reactions, stoichiometry, and the very nature of matter transformation. This article will provide a comprehensive explanation, exploring the nuances of reactants and products, their roles in various reaction types, and their importance in numerous scientific fields.
Reactants: The Starting Materials
Reactants are the starting materials in a chemical reaction. They are the substances that undergo a chemical change, transforming into new substances known as products. Think of them as the ingredients in a recipe – you need them to create the final dish. In a chemical equation, reactants are written on the left-hand side of the arrow.
Identifying Reactants:
Identifying reactants requires understanding the context of the reaction. Look for the substances that are consumed during the reaction. This consumption may be apparent through a change in physical properties (like color, temperature, or state of matter) or through the formation of entirely new substances.
Examples of Reactants:
- Combustion of Methane: In the combustion of methane (CH₄) with oxygen (O₂), both methane and oxygen are the reactants. They react to produce carbon dioxide and water.
- Neutralization Reaction: In a neutralization reaction between an acid (like hydrochloric acid, HCl) and a base (like sodium hydroxide, NaOH), both the acid and the base are reactants. They react to produce a salt and water.
- Photosynthesis: In photosynthesis, carbon dioxide (CO₂) and water (H₂O) are reactants. They are consumed by plants to produce glucose (C₆H₁₂O₆) and oxygen (O₂).
Characteristics of Reactants:
- Undergo Chemical Change: Reactants undergo a fundamental alteration in their chemical composition during the reaction. The bonds between atoms are broken and reformed, resulting in the creation of new substances.
- Limiting and Excess Reactants: In many reactions, one reactant is completely consumed before the others. This is called the limiting reactant. The other reactants present in excess are called excess reactants. The amount of product formed is determined by the limiting reactant.
- Reactant Concentration: The concentration of reactants significantly influences the reaction rate. Higher concentrations generally lead to faster reaction rates.
Products: The Result of the Reaction
Products are the new substances formed as a result of a chemical reaction. They are the outcome of the chemical transformation of the reactants. In a chemical equation, products are written on the right-hand side of the arrow.
Identifying Products:
Identifying products involves observing the changes occurring during the reaction. Look for the appearance of new substances with different physical and chemical properties compared to the reactants. This might involve the formation of a precipitate (a solid that separates from a solution), the evolution of a gas, or a significant change in color or temperature.
Examples of Products:
- Combustion of Methane (continued): In the combustion of methane, carbon dioxide (CO₂) and water (H₂O) are the products.
- Neutralization Reaction (continued): In a neutralization reaction, the salt (e.g., sodium chloride, NaCl) and water (H₂O) are the products.
- Photosynthesis (continued): In photosynthesis, glucose (C₆H₁₂O₆) and oxygen (O₂) are the products.
Characteristics of Products:
- New Substances: Products possess different chemical compositions and properties compared to the reactants.
- Formation Determined by Reactants: The type and amount of products formed are determined entirely by the types and amounts of reactants involved, as well as the reaction conditions.
- Yield: The actual amount of product obtained in a reaction is called the actual yield. The theoretical amount calculated based on stoichiometry is the theoretical yield. The percentage yield indicates the efficiency of the reaction.
The Chemical Equation: A Visual Representation
A chemical equation provides a concise and symbolic representation of a chemical reaction. It shows the reactants on the left-hand side, the products on the right-hand side, and the stoichiometric ratios between them. The arrow signifies the transformation from reactants to products.
For example, the combustion of methane is represented as:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation:
- CH₄ and 2O₂ are the reactants.
- CO₂ and 2H₂O are the products.
- The coefficients (numbers in front of the chemical formulas) indicate the stoichiometric ratios – one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
Types of Chemical Reactions and Reactants & Products
Different types of chemical reactions involve various reactants and products. Understanding these reaction types helps in predicting the products formed and analyzing the reaction mechanisms.
1. Synthesis Reactions (Combination Reactions):
In synthesis reactions, two or more reactants combine to form a single product. For example:
2H₂ + O₂ → 2H₂O (Hydrogen and oxygen react to form water)
2. Decomposition Reactions:
In decomposition reactions, a single reactant breaks down into two or more products. For example:
2H₂O₂ → 2H₂O + O₂ (Hydrogen peroxide decomposes into water and oxygen)
3. Single Displacement Reactions (Single Replacement Reactions):
In single displacement reactions, a more reactive element replaces a less reactive element in a compound. For example:
Zn + 2HCl → ZnCl₂ + H₂ (Zinc replaces hydrogen in hydrochloric acid)
4. Double Displacement Reactions (Double Replacement Reactions):
In double displacement reactions, two compounds exchange ions to form two new compounds. For example:
AgNO₃ + NaCl → AgCl + NaNO₃ (Silver nitrate and sodium chloride react to form silver chloride and sodium nitrate)
5. Combustion Reactions:
Combustion reactions involve the rapid reaction of a substance with oxygen, usually producing heat and light. For example:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O (Propane reacts with oxygen to form carbon dioxide and water)
6. Acid-Base Reactions (Neutralization Reactions):
Acid-base reactions involve the reaction between an acid and a base, usually producing a salt and water. For example:
HCl + NaOH → NaCl + H₂O (Hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water)
The Importance of Understanding Reactants and Products
The ability to distinguish between reactants and products is crucial in various fields:
- Chemistry: Fundamental to understanding chemical reactions, stoichiometry, reaction rates, and equilibrium.
- Biochemistry: Crucial for understanding metabolic pathways, enzyme function, and the synthesis and breakdown of biomolecules.
- Environmental Science: Understanding chemical reactions helps in analyzing pollution, remediation processes, and environmental impact assessments.
- Medicine: Essential for understanding drug mechanisms, metabolism, and drug interactions.
- Materials Science: Understanding chemical reactions is vital for designing and synthesizing new materials.
Conclusion
The distinction between reactants and products is a cornerstone of chemistry. This article has provided an in-depth exploration of their definitions, characteristics, roles in various reaction types, and their significance across multiple scientific disciplines. By mastering this fundamental concept, you lay a solid foundation for further exploration of the fascinating world of chemistry and its applications. Remember, understanding the transformation from reactants to products is key to understanding the very essence of chemical change.
Latest Posts
Latest Posts
-
Standard Enthalpy Of Formation For O2
Mar 29, 2025
-
How To Determine If A Reaction Is Spontaneous
Mar 29, 2025
-
What Bonds Are The Most Polar
Mar 29, 2025
-
Systems Of Linear Equations And Inequalities
Mar 29, 2025
-
Cuanto Pesa Un Galon De Agua
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Reactant And A Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
