What Is The Difference Between Chemical Reaction And Nuclear Reaction
Muz Play
Mar 18, 2025 · 6 min read
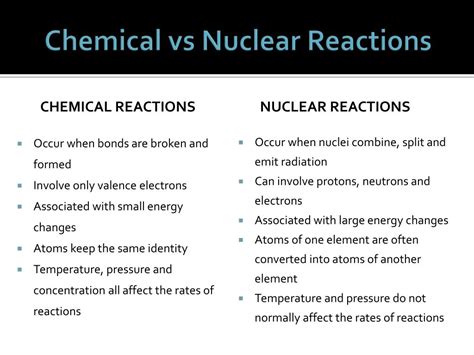
Table of Contents
Delving Deep: Chemical Reactions vs. Nuclear Reactions
Understanding the fundamental differences between chemical reactions and nuclear reactions is crucial for grasping the complexities of the physical world. While both involve transformations of matter, they operate at drastically different scales and involve different fundamental forces. This comprehensive guide will dissect these differences, exploring the intricacies of each process and highlighting their key distinctions.
The Realm of Chemical Reactions: Rearranging Atoms
Chemical reactions involve the rearrangement of atoms to form new molecules. These reactions primarily affect the valence electrons, the outermost electrons of an atom that participate in bonding. The nuclei of the atoms remain unchanged. Chemical reactions are governed by the electromagnetic force, the force responsible for interactions between charged particles.
Key Characteristics of Chemical Reactions:
- Relatively Low Energy Changes: Chemical reactions typically involve relatively small energy changes, often measured in kilojoules (kJ) or kilocalories (kcal). This energy is absorbed or released during the breaking and formation of chemical bonds.
- Changes in Chemical Properties: Chemical reactions result in changes in the chemical properties of the substances involved. The reactants transform into entirely different products with distinct physical and chemical characteristics. For example, the reaction between hydrogen and oxygen to form water results in a significant change in properties: from highly reactive gases to a stable liquid.
- No Change in Atomic Nuclei: A defining feature of chemical reactions is that the atomic nuclei of the elements remain unaltered. The same atoms are present before and after the reaction; they simply rearrange themselves into different combinations.
- Observable Changes: Many chemical reactions are accompanied by observable changes such as color change, temperature change, gas evolution, or precipitate formation. These changes provide visual evidence of the reaction taking place.
- Rate influenced by factors such as temperature, pressure, and catalysts: The speed or rate of a chemical reaction can be significantly influenced by external factors such as temperature, pressure, concentration of reactants, and the presence of catalysts. Catalysts accelerate reactions without being consumed themselves.
Examples of Chemical Reactions:
- Combustion: The rapid reaction between a substance and an oxidant (typically oxygen) producing heat and light. Burning wood or natural gas are everyday examples.
- Neutralization: The reaction between an acid and a base, resulting in the formation of a salt and water. The neutralization of stomach acid with antacids is a common example.
- Photosynthesis: The process by which plants convert light energy into chemical energy in the form of glucose. This is a complex series of chemical reactions.
- Rusting: The slow oxidation of iron in the presence of oxygen and water, leading to the formation of iron oxide (rust).
The Realm of Nuclear Reactions: Manipulating the Nucleus
Nuclear reactions, in stark contrast to chemical reactions, involve changes within the atomic nucleus. These reactions are far more energetic and result in the transformation of one element into another. Nuclear reactions are governed by the strong nuclear force, which holds protons and neutrons together within the nucleus, and the weak nuclear force, responsible for certain types of radioactive decay.
Key Characteristics of Nuclear Reactions:
- Extremely High Energy Changes: Nuclear reactions involve enormous energy changes, often measured in megajoules (MJ) or even gigajoules (GJ) per mole of reactant. This vast energy release stems from the conversion of a small amount of mass into energy, as described by Einstein's famous equation, E=mc².
- Transmutation of Elements: A hallmark of nuclear reactions is the transmutation of elements. This means that the atomic number (number of protons) of the nucleus changes, resulting in the formation of a different element. For instance, the radioactive decay of uranium-238 produces thorium-234.
- Changes in Atomic Nuclei: Unlike chemical reactions, nuclear reactions involve significant changes within the atomic nucleus. Protons and neutrons can be added, removed, or rearranged, leading to changes in the element's identity.
- Radioactive Decay: Many nuclear reactions involve radioactive decay, a spontaneous process where unstable atomic nuclei emit particles (alpha, beta, or gamma radiation) to become more stable.
- Nuclear Fission and Fusion: These are two major types of nuclear reactions. Fission involves the splitting of a heavy nucleus into two lighter nuclei, while fusion involves the combining of light nuclei to form a heavier nucleus. Both processes release tremendous amounts of energy.
- Rate not significantly influenced by temperature and pressure: Unlike chemical reactions, the rate of nuclear reactions is largely unaffected by changes in temperature or pressure.
Examples of Nuclear Reactions:
- Nuclear Fission: The splitting of a uranium or plutonium nucleus by neutron bombardment, releasing a large amount of energy and more neutrons. This process is used in nuclear power plants and atomic bombs.
- Nuclear Fusion: The combining of light atomic nuclei, such as isotopes of hydrogen (deuterium and tritium), at extremely high temperatures and pressures to form helium, releasing immense energy. This process powers the sun and stars.
- Radioactive Decay: The spontaneous breakdown of unstable atomic nuclei, emitting particles or energy. Examples include the decay of carbon-14 used in radiocarbon dating and the decay of uranium used in geological dating.
- Nuclear Transmutation: The artificial conversion of one element into another through bombardment with particles like protons or neutrons. This process is used to create new isotopes and elements in laboratories.
A Table Summarizing the Key Differences:
| Feature | Chemical Reaction | Nuclear Reaction |
|---|---|---|
| Energy Change | Relatively low (kJ/kcal) | Extremely high (MJ/GJ) |
| Atoms Involved | Valence electrons only | Protons and neutrons in the nucleus |
| Elements | No change in elemental identity | Transmutation of elements |
| Forces Involved | Electromagnetic force | Strong and weak nuclear forces |
| Rate Influence | Temperature, pressure, catalysts | Largely unaffected by temperature/pressure |
| Mass Change | Negligible | Measurable (E=mc²) |
| Examples | Combustion, neutralization, rusting | Fission, fusion, radioactive decay |
Beyond the Basics: Exploring the Interplay
While distinct, chemical and nuclear reactions aren't entirely isolated phenomena. There can be interactions and overlaps:
- Chemical reactions can initiate or be influenced by nuclear processes: For example, the chemical reactivity of an element can vary significantly depending on its isotopic composition (different numbers of neutrons). Radioactive decay products can also trigger further chemical reactions.
- Nuclear reactions can induce chemical changes: The high energy released during nuclear reactions can lead to significant changes in the chemical environment, such as ionization and the formation of new chemical species. This is particularly relevant in nuclear explosions and radiation chemistry.
- Chemical processes can be used to separate isotopes: This is crucial in nuclear technologies. Techniques like gas diffusion and centrifugation exploit subtle differences in the chemical behavior of isotopes to enrich or separate them.
Conclusion: Understanding the Fundamental Forces
The fundamental difference between chemical and nuclear reactions lies in the level at which they operate. Chemical reactions involve the rearrangement of atoms through changes in their valence electrons, while nuclear reactions involve changes within the atomic nucleus itself. This difference leads to dramatic variations in energy changes, the types of forces involved, and the ultimate outcome of the process. Understanding these distinctions is essential for comprehending a wide range of phenomena, from the processes of life to the power of the sun and the potential dangers of nuclear weapons. The continued study of both chemical and nuclear reactions will undoubtedly continue to reveal new insights into the fundamental forces that govern our universe.
Latest Posts
Latest Posts
-
Converting Double Integrals To Polar Coordinates
Mar 19, 2025
-
How Do You Convert Moles To Volume
Mar 19, 2025
-
The Horizontal Columns On The Periodic Table Are Called
Mar 19, 2025
-
How To Find Derivative Of Limit
Mar 19, 2025
-
Proof Of The Parallel Axis Theorem
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Chemical Reaction And Nuclear Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
