What Is The Difference Between Heat Capacity And Specific Heat
Muz Play
Mar 19, 2025 · 6 min read
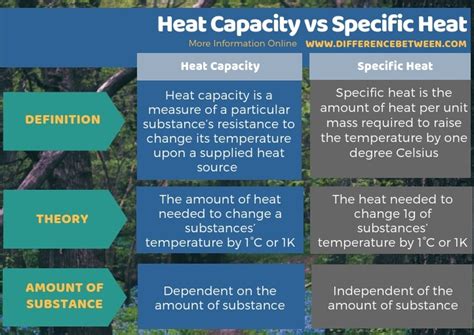
Table of Contents
What's the Difference Between Heat Capacity and Specific Heat? A Deep Dive
Understanding the difference between heat capacity and specific heat is crucial for anyone studying thermodynamics, material science, or engineering. While both concepts relate to how much heat an object absorbs or releases with a change in temperature, they differ significantly in their scope and application. This article will delve deep into the nuances of both, providing clear explanations, practical examples, and highlighting the key distinctions.
Defining Heat Capacity: The Big Picture
Heat capacity, denoted by 'C', represents the amount of heat energy required to raise the temperature of a substance by one degree Celsius (or one Kelvin). It's a measure of a substance's ability to store thermal energy. The crucial point here is that heat capacity refers to the entire object or system under consideration, encompassing its total mass. A large block of iron will have a far greater heat capacity than a small iron nail, simply because it contains more atoms and therefore can absorb more heat for the same temperature change.
The formula for heat capacity is:
C = Q/ΔT
Where:
- C is the heat capacity (measured in Joules per Kelvin, J/K or Joules per degree Celsius, J/°C)
- Q is the amount of heat energy transferred (measured in Joules, J)
- ΔT is the change in temperature (measured in Kelvin or degrees Celsius)
Understanding the Implications of Heat Capacity
The heat capacity of an object is influenced by several factors:
- Mass: As mentioned earlier, a larger mass generally leads to a larger heat capacity. More material means more energy is required for a temperature increase.
- Material Composition: Different substances have vastly different atomic structures and bonding characteristics. These microscopic details significantly influence how effectively a material can absorb and store thermal energy. For instance, water has a remarkably high heat capacity compared to most other substances.
- Phase: The state of matter (solid, liquid, or gas) also plays a role. Generally, the heat capacity of a substance changes when it transitions between phases (e.g., melting ice to liquid water).
- Temperature: While often considered constant within a temperature range, heat capacity can slightly vary with temperature. This variation is usually negligible for many practical applications but becomes important in high-precision measurements and extreme temperature conditions.
Defining Specific Heat: Focusing on the Intrinsic Property
Specific heat, often denoted by 'c', is a fundamental property of a material that quantifies the amount of heat required to raise the temperature of one unit mass of the substance by one degree Celsius (or one Kelvin). Unlike heat capacity, specific heat is an intensive property, meaning it is independent of the amount of substance present. A small piece of copper will have the same specific heat as a large copper block. This makes it a far more valuable constant for material characterization.
The formula for specific heat is:
c = Q/(mΔT)
Where:
- c is the specific heat (measured in Joules per kilogram-Kelvin, J/kg·K or Joules per kilogram-degree Celsius, J/kg·°C)
- Q is the amount of heat energy transferred (measured in Joules, J)
- m is the mass of the substance (measured in kilograms, kg)
- ΔT is the change in temperature (measured in Kelvin or degrees Celsius)
Specific Heat: A Material Constant
The specific heat is an intrinsic property, meaning it's specific to the material itself. This means:
- Constant for a given substance: The specific heat of water, for example, is approximately 4186 J/kg·K, regardless of the quantity of water.
- Useful for comparison: It allows for easy comparison of the heat-absorbing capacities of different materials. Materials with higher specific heat require more energy to raise their temperature.
- Essential in engineering and science: Accurate specific heat data is essential in countless applications, including designing thermal systems, understanding material behavior, and modeling heat transfer processes.
Key Differences: A Summary Table
| Feature | Heat Capacity (C) | Specific Heat (c) |
|---|---|---|
| Definition | Heat required to raise the temperature of an object by 1°C/1K | Heat required to raise the temperature of 1kg of a substance by 1°C/1K |
| Type of property | Extensive (depends on mass) | Intensive (independent of mass) |
| Units | J/°C or J/K | J/kg·°C or J/kg·K |
| Application | Analyzing entire systems | Characterizing material properties |
| Dependence on mass | Directly proportional to mass | Independent of mass |
Practical Examples to Illustrate the Difference
Let's consider two examples to solidify the distinction:
Example 1: Heating a Copper Block
Imagine you have two copper blocks: one weighing 1 kg and the other weighing 2 kg. Both have the same specific heat (approximately 385 J/kg·K). If you apply the same amount of heat to both blocks, the smaller block will experience a larger temperature increase because it has a smaller heat capacity. The specific heat remains constant throughout.
Example 2: Comparing Water and Iron
Let's compare heating equal masses of water and iron. Water has a much higher specific heat than iron (approximately 4186 J/kg·K for water vs. approximately 450 J/kg·K for iron). To raise the temperature of 1 kg of water by 1°C, you need significantly more heat energy than to raise the temperature of 1 kg of iron by the same amount. This difference is reflected in their specific heats. However, a larger volume of iron would have a higher heat capacity than a smaller volume of water, even though the water has a higher specific heat.
Advanced Concepts and Applications
The understanding of heat capacity and specific heat extends beyond basic thermodynamics. Here are a few advanced applications:
- Calorimetry: The determination of specific heat often involves calorimetric techniques, which precisely measure the heat transfer during a process.
- Phase Transitions: The specific heat changes dramatically during phase transitions (melting, boiling, etc.), reflecting the energy required to break or form intermolecular bonds.
- Heat Transfer Calculations: Accurate values for specific heat are crucial for modeling and predicting heat transfer in various engineering systems, from HVAC systems to power plants.
- Material Science: The specific heat of a material provides insights into its atomic structure, bonding characteristics, and thermal properties.
Conclusion: Two Sides of the Same Coin
Heat capacity and specific heat are closely related concepts, both describing the thermal behavior of substances. However, heat capacity considers the total heat required for a given temperature change in an object, while specific heat focuses on the intrinsic property of a material, irrespective of its mass. Understanding both concepts is crucial for mastering thermodynamics and tackling numerous applications across various scientific and engineering disciplines. The distinction between these two concepts is essential for accurate calculations and a deeper comprehension of thermal processes. By appreciating both their similarities and their differences, you gain a comprehensive understanding of how heat interacts with matter.
Latest Posts
Latest Posts
-
How Do You Find The Rate Determining Step
Mar 19, 2025
-
What Is A Two Letter Symbol From The Periodic Table
Mar 19, 2025
-
How Many Nadh Produced In Glycolysis
Mar 19, 2025
-
Avg Power In Rlc Circuit Fomrula
Mar 19, 2025
-
The Language Of Anatomy Exercise 1
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Heat Capacity And Specific Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
