What Is A Two Letter Symbol From The Periodic Table
Muz Play
Mar 19, 2025 · 5 min read
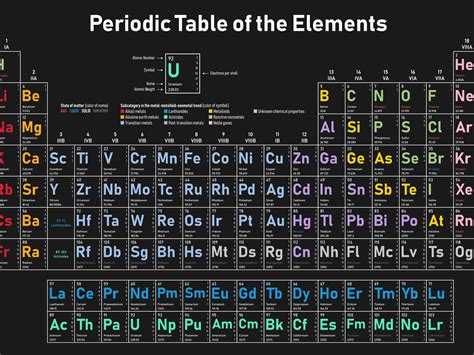
Table of Contents
What is a Two-Letter Symbol from the Periodic Table? A Deep Dive into Chemical Nomenclature
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While most elements boast one-letter or two-letter symbols, understanding the why behind these abbreviations – especially the two-letter ones – reveals a fascinating history and intricate system of chemical nomenclature. This article delves deep into the world of two-letter element symbols, exploring their origins, the reasons behind their existence, and the implications for chemical notation.
The Genesis of Chemical Symbols: From Alchemists to Mendeleev
Before the standardized symbols we know today, alchemy held sway. Alchemists used various symbols, often cryptic and personalized, to represent elements and processes. These weren't standardized, leading to considerable confusion. The shift towards a more systematic approach began with the growing understanding of atomic weights and the emergence of early periodic table iterations. Jöns Jacob Berzelius, a Swedish chemist, played a pivotal role in modernizing chemical notation. His system, introducing letter-based abbreviations derived from element names (mostly Latin or Greek), provided a much-needed level of consistency.
Why Two-Letter Symbols? Addressing Ambiguity and Expanding the Table
The use of two-letter symbols primarily arose from the necessity to differentiate elements. As the periodic table expanded beyond the initial twenty-six elements, a single letter for each was no longer sufficient. Imagine the confusion if both Sodium (Na) and Nitrogen (N) were assigned just one letter – 'N'! Two-letter symbols elegantly resolve this ambiguity, providing a unique identifier for every element discovered, whether naturally occurring or synthetically created.
Examples of Two-Letter Symbols and their Origins
Let's examine some notable examples to understand the rationale behind these two-letter choices:
-
He (Helium): A straightforward case, deriving directly from its name. This brevity reflects its common usage and early discovery.
-
Ne (Neon): Similarly, Neon's symbol seamlessly reflects its name, maintaining clarity and consistency.
-
Ar (Argon): Argon's symbol follows the established pattern, utilizing the initial two letters of its name.
-
Si (Silicon): Silicon's symbol 'Si' originates from the Latin word "silicium," highlighting the link between modern naming conventions and historical influences.
-
Cl (Chlorine): Chlorine's symbol "Cl" is derived from its Greek-rooted name "chloros," meaning "greenish-yellow." This links its chemical symbol to its distinctive color.
-
Br (Bromine): Bromine's "Br" symbol comes from the Greek word "bromos," meaning "stench," reflecting its pungent odor. This illustrates how symbols sometimes connect to physical characteristics.
-
Kr (Krypton): Krypton, a noble gas, received its symbol 'Kr' directly from its name, maintaining simplicity and consistency.
-
Xe (Xenon): Xenon's 'Xe' neatly reflects the name, further solidifying the trend of utilizing the first two letters of an element's name for its chemical symbol.
-
Rn (Radon): Radon's symbol 'Rn' directly follows from its name, maintaining a clear and simple representation.
-
Zn (Zinc): While zinc's modern name seems unrelated to "Zn," its symbol traces back to the German word "Zink," highlighting the evolution of naming conventions across languages.
The Importance of Consistent Nomenclature
The consistency provided by two-letter (and one-letter) symbols is crucial for effective communication within the scientific community. Imagine the chaos if different laboratories or publications used varied symbols for the same element! This standardized system ensures that chemical formulas, equations, and research findings are universally understood, fostering collaboration and accelerating scientific progress.
Beyond the Symbols: Understanding Atomic Numbers
While symbols are crucial for brevity and recognition, understanding the atomic number is fundamental. Each element possesses a unique atomic number, representing the number of protons in its nucleus. This number is independent of the symbol; it's the element's fundamental identity.
Two-Letter Symbols and Isotopes
Isotopes are atoms of the same element with varying neutron counts. While the element's symbol remains unchanged (e.g., always 'C' for Carbon), the difference in neutrons affects the atom's mass number. This understanding is vital for nuclear chemistry and related fields.
Two-Letter Symbols in Chemical Equations and Formulas
Two-letter symbols are indispensable components of chemical equations and formulas. They allow scientists to represent reactants, products, and stoichiometric relationships concisely. The consistent use of these symbols facilitates calculations, predictions of reaction outcomes, and understanding reaction mechanisms.
The Future of Chemical Nomenclature
As the quest for new elements continues, the current system of one and two-letter symbols is robust enough to handle any future discoveries. The established conventions ensure clarity and prevent ambiguities, making the system remarkably efficient and adaptable to ongoing scientific advancements.
Practical Applications of Two-Letter Symbols
Two-letter element symbols are not confined to theoretical chemical discussions. They have tangible and crucial applications in several fields, including:
-
Materials Science: Designing new materials relies on understanding the properties of constituent elements, indicated by their symbols. The use of symbols facilitates efficient communication and research.
-
Pharmaceutical Research: Drug discovery and development necessitate careful chemical representation. Symbols are crucial for denoting molecular structures and predicting drug interactions.
-
Environmental Science: Analyzing environmental samples, identifying pollutants, and understanding chemical processes all hinge on the standardized representation of elements through their symbols.
-
Industrial Chemistry: Various industrial processes depend on precise chemical compositions and reactions. Symbols ensure clarity and accuracy in production and quality control.
-
Nuclear Science: Understanding nuclear reactions, isotope behavior, and radioactive decay utilizes symbols in a fundamental way. This area necessitates precise notation for safety and accuracy.
Conclusion: A Powerful System of Chemical Communication
The use of two-letter symbols within the periodic table demonstrates an elegant solution to a complex problem – the need for a universally understood and unambiguous system for representing chemical elements. From the evolution of alchemic symbols to the standardized notations we use today, the development of chemical nomenclature showcases the collaborative effort of scientists to create a universally accessible language of chemistry. The continued use and importance of these two-letter symbols underscores their enduring significance in scientific communication and research across various disciplines. The simplicity and efficiency of the system ensure its continued relevance in a constantly evolving scientific landscape. The precise and concise nature of these symbols underpins the very foundation of chemical understanding and lays the groundwork for future discoveries.
Latest Posts
Latest Posts
-
Is Cl A Good Leaving Group
Mar 20, 2025
-
Shaft Of Long Bone Is Called
Mar 20, 2025
-
Differential Equations Method Of Variation Of Parameters
Mar 20, 2025
-
Is Naoh A Weak Or Strong Base
Mar 20, 2025
-
Oh Once One Takes The Anatomy Final
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is A Two Letter Symbol From The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
