Is Naoh A Weak Or Strong Base
Muz Play
Mar 20, 2025 · 5 min read
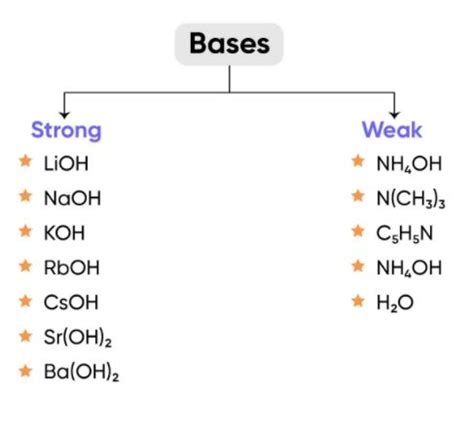
Table of Contents
Is NaOH a Weak or Strong Base? A Comprehensive Guide
Sodium hydroxide (NaOH), also known as lye or caustic soda, is a ubiquitous chemical compound with diverse applications in various industries. Understanding its chemical properties, particularly its basicity, is crucial for safe and effective handling. This comprehensive guide delves deep into the question: Is NaOH a weak or strong base? The answer, unequivocally, is strong. But let's explore the reasons behind this classification and examine its implications.
Understanding Acids and Bases
Before we definitively categorize NaOH, let's establish a foundational understanding of acids and bases. Several theories exist to explain their behavior, but two prominent models are the Arrhenius and Brønsted-Lowry theories.
Arrhenius Theory
The Arrhenius theory defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, and bases as substances that produce hydroxide ions (OH⁻) in water. While simple, this model is limited in its scope, failing to explain the behavior of bases that don't contain hydroxide ions.
Brønsted-Lowry Theory
The Brønsted-Lowry theory offers a broader perspective. It defines acids as proton (H⁺) donors and bases as proton acceptors. This theory expands the definition of bases beyond just hydroxide-containing substances, encompassing a wider range of compounds.
Strong vs. Weak Bases
The distinction between strong and weak bases lies in their degree of dissociation in water.
Strong Bases
Strong bases completely dissociate into their constituent ions in aqueous solutions. This means that virtually every molecule of the base releases its hydroxide ions (OH⁻) when dissolved in water. This complete dissociation results in a high concentration of hydroxide ions, leading to a high pH.
Weak Bases
Weak bases, on the other hand, only partially dissociate in water. A significant portion of the weak base molecules remain undissociated, resulting in a lower concentration of hydroxide ions compared to a strong base at the same concentration. This partial dissociation leads to a lower pH than a strong base of equivalent concentration.
NaOH: A Strong Base Explained
NaOH, upon dissolving in water, undergoes complete dissociation:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
This equation clearly shows that each molecule of NaOH releases one sodium ion (Na⁺) and one hydroxide ion (OH⁻). The complete dissociation is the hallmark of a strong base. There's no equilibrium established between the undissociated NaOH and its ions; it's a one-way reaction.
The Significance of Complete Dissociation
The complete dissociation of NaOH has significant implications:
- High pH: The high concentration of OH⁻ ions leads to a highly alkaline solution with a pH significantly greater than 7. The exact pH depends on the concentration of the NaOH solution.
- High Reactivity: The high concentration of OH⁻ ions makes NaOH highly reactive with various substances, including acids, metals, and organic compounds. This reactivity is exploited in various industrial applications.
- Conductivity: The complete dissociation into ions makes NaOH solutions excellent conductors of electricity. This property is utilized in various electrochemical processes.
Comparing NaOH to Weak Bases
Let's contrast NaOH's behavior with that of a weak base, such as ammonia (NH₃). Ammonia only partially dissociates in water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Notice the equilibrium arrows (⇌). This indicates that the reaction proceeds in both directions simultaneously. A significant portion of ammonia remains undissociated in the solution, resulting in a much lower concentration of OH⁻ ions compared to an equivalent concentration of NaOH. This difference in dissociation is the fundamental reason why NaOH is classified as a strong base and ammonia as a weak base.
Practical Implications of NaOH's Strength
The strong base nature of NaOH has several practical consequences, both beneficial and hazardous:
Beneficial Applications
- Industrial Cleaning: NaOH is a powerful cleaning agent used in various industrial settings to remove grease, oil, and other contaminants.
- Chemical Synthesis: It plays a crucial role as a reactant and catalyst in numerous chemical syntheses, particularly in the production of soaps, detergents, and various chemicals.
- Pulp and Paper Industry: NaOH is essential in the production of paper pulp from wood.
- Food Industry: Used in food processing, albeit with strict regulations, for tasks such as peeling fruits and vegetables.
- Drain Cleaners: Many commercial drain cleaners contain NaOH to dissolve organic clogs.
Hazards Associated with NaOH
The strong reactivity of NaOH poses significant safety concerns:
- Caustic Burns: Direct contact with NaOH can cause severe chemical burns to skin, eyes, and mucous membranes. Immediate and thorough rinsing with water is crucial in case of accidental contact.
- Exothermic Reactions: Reactions involving NaOH are often exothermic, releasing significant heat. This can lead to burns or fires if not properly managed.
- Environmental Impact: Improper disposal of NaOH can harm the environment, affecting soil and water quality.
Identifying NaOH as a Strong Base: Experimental Evidence
Several experiments can demonstrate NaOH's strong base nature:
- pH Measurement: Using a pH meter or indicator solutions, we can easily determine the high pH of an NaOH solution, confirming its strong base character.
- Titration: Titration with a standard acid allows precise determination of the concentration of OH⁻ ions, supporting the complete dissociation of NaOH.
- Conductivity Measurement: The high electrical conductivity of NaOH solutions further confirms its complete dissociation into ions.
Conclusion: NaOH – An Unmistakably Strong Base
In conclusion, NaOH is undeniably a strong base. Its complete dissociation in water, resulting in a high concentration of hydroxide ions, high pH, and high reactivity, differentiates it from weak bases that only partially dissociate. Understanding this fundamental property is essential for safe and effective handling and utilization of this crucial chemical compound across various industries. Its powerful properties, while beneficial in many applications, necessitate careful handling and adherence to strict safety protocols to mitigate potential hazards. Always prioritize safety when working with NaOH or any other strong base.
Latest Posts
Latest Posts
-
Mole Ratios For A Reaction Are Obtained From The
Mar 20, 2025
-
How Does An Electrolyte Conduct Electricity
Mar 20, 2025
-
What Are The Characteristics Of Population
Mar 20, 2025
-
How Many Copies Of Dna Are Generated After 30 Cycles
Mar 20, 2025
-
What Is The Difference Between Real And Ideal Values
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Naoh A Weak Or Strong Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
