How Does An Electrolyte Conduct Electricity
Muz Play
Mar 20, 2025 · 6 min read
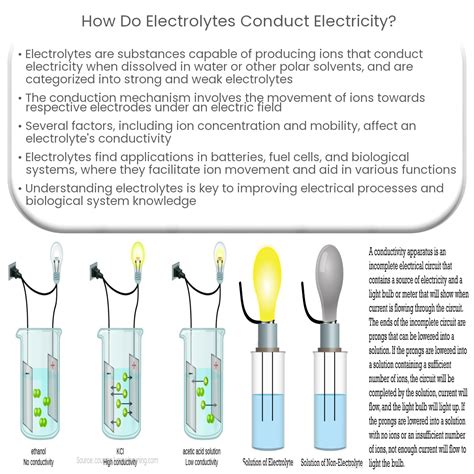
Table of Contents
How Does an Electrolyte Conduct Electricity?
Electrolytes are substances that, when dissolved in a solvent or melted, conduct electricity. This seemingly simple statement belies a fascinating and complex process involving the movement of charged particles. Understanding how electrolytes conduct electricity is fundamental to numerous fields, including electrochemistry, biology, and materials science. This comprehensive article will delve into the intricacies of electrolytic conduction, exploring the different types of electrolytes, the mechanisms behind their conductivity, and the factors that influence their effectiveness.
The Role of Ions in Electrical Conduction
The key to an electrolyte's ability to conduct electricity lies in the presence of ions. Unlike metallic conductors where electrons are the primary charge carriers, electrolytes conduct electricity through the movement of both positive cations and negative anions. These ions are formed when an electrolyte dissolves or melts, a process often involving the dissociation of molecules or the breaking apart of an ionic lattice.
Dissociation and Ionization
Dissociation refers to the separation of existing ions in an ionic compound when dissolved in a suitable solvent. For example, when sodium chloride (NaCl) dissolves in water, it dissociates into sodium cations (Na⁺) and chloride anions (Cl⁻). The polar water molecules surround and stabilize these ions, preventing them from recombining.
Ionization, on the other hand, is the process where neutral molecules gain or lose electrons to form ions. This often occurs in solutions containing weak acids or bases. For instance, acetic acid (CH₃COOH) partially ionizes in water to produce acetate anions (CH₃COO⁻) and hydronium cations (H₃O⁺). The extent of ionization depends on the strength of the acid or base and the solvent's properties.
Types of Electrolytes
Electrolytes are broadly classified into several categories based on their properties and behavior:
Strong vs. Weak Electrolytes
Strong electrolytes completely dissociate or ionize in solution, resulting in high conductivity. Examples include strong acids (like HCl, HNO₃, H₂SO₄), strong bases (like NaOH, KOH), and most soluble salts (like NaCl, KCl). Every molecule essentially breaks apart into its constituent ions.
Weak electrolytes, conversely, only partially dissociate or ionize in solution, leading to lower conductivity. Weak acids (like acetic acid, CH₃COOH), weak bases (like ammonia, NH₃), and some sparingly soluble salts fall into this category. An equilibrium exists between the undissociated molecules and the ions.
Molten Electrolytes
Electrolytes don't necessarily require a solvent. Molten electrolytes are ionic compounds in their liquid state, often achieved by heating them above their melting points. In this state, the ions are free to move and conduct electricity. The production of aluminum through the Hall-Héroult process, for example, relies on molten cryolite as an electrolyte.
Factors Affecting Electrolytic Conductivity
Several factors influence how effectively an electrolyte conducts electricity:
Concentration
The concentration of ions directly impacts conductivity. Higher concentrations generally lead to higher conductivity because there are more charge carriers available to move and carry the current. However, this relationship isn't always strictly linear; at very high concentrations, interionic interactions can hinder ion mobility, reducing conductivity.
Temperature
Temperature significantly influences conductivity. Increasing the temperature typically enhances conductivity in both aqueous and molten electrolytes. Higher temperatures increase the kinetic energy of ions, leading to faster movement and thus greater current flow. The increased thermal agitation also overcomes some of the interionic forces, further promoting ion mobility.
Solvent Properties
The solvent plays a crucial role in electrolytic conductivity. The solvent's polarity, dielectric constant, and viscosity all affect ion solvation and mobility. Polar solvents, such as water, effectively solvate ions, reducing interionic attractions and promoting conductivity. High dielectric constants further enhance ion separation. Conversely, viscous solvents hinder ion movement, reducing conductivity.
Ion Size and Charge
The size and charge of the ions also influence conductivity. Smaller ions generally move faster than larger ions due to less resistance from the solvent. Similarly, ions with higher charges contribute more to the current flow than those with lower charges, given their stronger electrostatic interactions.
Mechanisms of Ionic Conduction
The movement of ions in an electrolyte isn't simply a random diffusion process. Several mechanisms contribute to ionic conduction:
Migration
Under the influence of an applied electric field, ions migrate towards the electrode with opposite charge. Cations move towards the cathode (negative electrode), while anions move towards the anode (positive electrode). This directed movement of ions is the primary mechanism of electrolytic conduction.
Diffusion
Even in the absence of an applied electric field, a concentration gradient can drive ion movement through diffusion. Ions naturally tend to move from regions of higher concentration to regions of lower concentration, contributing to overall ionic movement and conductivity.
Convection
In some cases, especially in liquid electrolytes, convection can contribute to ion transport. This involves bulk fluid movement, driven by factors like temperature gradients or stirring, which can enhance the distribution and movement of ions.
Applications of Electrolytic Conduction
The principle of electrolytic conduction underlies a vast array of applications:
Batteries and Fuel Cells
Batteries and fuel cells rely on the movement of ions within an electrolyte to generate electricity. The electrolyte acts as a medium for ion transport between the electrodes, completing the electrical circuit. Different types of electrolytes are employed depending on the specific battery or fuel cell chemistry.
Electroplating and Electrorefining
Electroplating involves using an electrolyte to deposit a metal layer onto a substrate. Electrorefining utilizes electrolytes to purify metals by selectively dissolving and redepositing them. These processes rely on the controlled movement of ions in the electrolyte.
Electrolysis
Electrolysis uses an electric current to drive non-spontaneous chemical reactions. The electrolyte provides the ionic medium for the passage of current and the participation of ions in the electrochemical reactions. Examples include the production of hydrogen and chlorine gas from water and the production of aluminum from alumina.
Biological Systems
Electrolytic conduction plays a critical role in many biological systems. Nerve impulses, for instance, rely on the movement of ions across cell membranes, generating electrical signals. Electrolyte balance in the body is essential for proper functioning of muscles, nerves, and other organs.
Sensors and Actuators
Electrolytic conductivity forms the basis of many sensors and actuators. Changes in conductivity, often related to changes in concentration or temperature, can be detected and used to monitor various parameters.
Conclusion
Electrolytic conduction is a complex process governed by several interconnected factors. Understanding the nature of ions, the role of the solvent, and the mechanisms of ionic transport is crucial for harnessing the power of electrolytes in various applications. As research continues to delve deeper into the intricacies of electrolytic behavior, we can expect to see even more innovative applications of this fundamental principle in the years to come. The continued development of new electrolytes with improved conductivity, stability, and safety will be critical in advancing numerous technologies and scientific endeavors. Further research in solid-state electrolytes, for example, holds promise for improving battery performance and safety. The ability to finely tune and control electrolytic conductivity is a key factor driving progress in fields ranging from energy storage to biosensors.
Latest Posts
Latest Posts
-
How To Find The Instantaneous Acceleration
Mar 20, 2025
-
Periodic Table Gases Liquids And Solids
Mar 20, 2025
-
The Dorsal Body Cavity Is Subdivided Into The
Mar 20, 2025
-
Allowance Method Vs Direct Write Off
Mar 20, 2025
-
Electrically Neutral Atoms Have Equal Numbers Of Electrons And Protons
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Does An Electrolyte Conduct Electricity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
