Is Cl A Good Leaving Group
Muz Play
Mar 20, 2025 · 6 min read
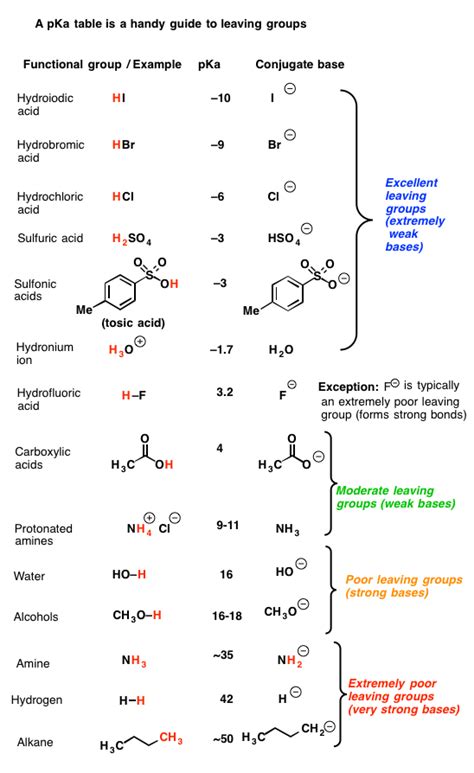
Table of Contents
Is Cl a Good Leaving Group? A Comprehensive Analysis
Chloride ion (Cl⁻), a common leaving group in organic chemistry reactions, often sparks debate about its effectiveness. While generally considered a good leaving group, its reactivity is context-dependent. This in-depth analysis explores the factors influencing Cl⁻'s leaving group ability, comparing it to other common leaving groups and examining its role in various reaction mechanisms. We'll delve into the intricacies of its behavior, providing a comprehensive understanding for students and professionals alike.
Understanding Leaving Groups: The Basics
Before we dive into the specifics of Cl⁻, let's establish a fundamental understanding of leaving groups. In organic chemistry, a leaving group (LG) is an atom or group of atoms that departs with a pair of electrons during a heterolytic bond cleavage. A good leaving group stabilizes the negative charge it acquires after leaving. This stability is crucial for the reaction to proceed efficiently.
Several factors determine the quality of a leaving group:
-
Stability of the conjugate base: A good leaving group forms a stable conjugate base. This stability is directly related to the ability of the leaving group to accommodate the negative charge. Weak bases are generally better leaving groups because they are more stable with the extra electrons.
-
Polarizability: Highly polarizable leaving groups are better because they can better disperse the negative charge.
-
Resonance stabilization: Leaving groups with resonance structures are more stable and thus better leaving groups.
-
Size and electronegativity: Larger and more electronegative atoms are generally better leaving groups because they can better handle the negative charge.
Assessing Chloride Ion (Cl⁻) as a Leaving Group
Chloride ion fits the profile of a good leaving group reasonably well. It's relatively stable due to its electronegativity and size. The conjugate acid, HCl, is a strong acid, indicating that Cl⁻ is a weak conjugate base. This weak basicity is a key characteristic of a good leaving group – it doesn't readily accept a proton back, facilitating the completion of the reaction.
However, it's crucial to remember that "good" is relative. Cl⁻'s performance is heavily influenced by the specific reaction conditions and the nature of the substrate. Let's analyze some scenarios where Cl⁻ excels and others where it might fall short.
Scenarios where Cl⁻ excels as a leaving group:
-
SN1 and SN2 Reactions: In nucleophilic substitution reactions, Cl⁻ performs reasonably well. In SN1 reactions (unimolecular nucleophilic substitution), the leaving group departs first to form a carbocation intermediate. The stability of this intermediate largely dictates the reaction rate. While Cl⁻ isn't the best leaving group for SN1 reactions (tosylates and triflates are preferred), it's competent, particularly with tertiary or stable secondary carbocations. In SN2 reactions (bimolecular nucleophilic substitution), the nucleophile attacks simultaneously with the departure of the leaving group. Cl⁻'s moderate size and electronegativity contribute to a reasonable reaction rate, although better leaving groups like iodides (I⁻) generally react faster.
-
Elimination Reactions (E1 and E2): Similar to substitution reactions, Cl⁻ functions adequately in elimination reactions. In E1 reactions (unimolecular elimination), the leaving group departs first, forming a carbocation, which then undergoes deprotonation to form the alkene. In E2 reactions (bimolecular elimination), the base abstracts a proton simultaneously with the departure of the leaving group. Again, while not the optimal choice (tosylates often outperform Cl⁻), it's a viable leaving group, particularly when strong bases are employed in E2 reactions.
Scenarios where Cl⁻ might not be the ideal leaving group:
-
Competition with other leaving groups: When compared to other halides, especially iodine (I⁻) and bromine (Br⁻), Cl⁻ is generally a weaker leaving group. Iodide, with its larger size and greater polarizability, is a much better leaving group because it stabilizes the negative charge more effectively. Bromide (Br⁻) also surpasses chloride in leaving group ability.
-
Steric hindrance: In reactions where steric hindrance is significant, Cl⁻'s relatively smaller size might not be advantageous. Bulky leaving groups can sometimes facilitate reactions by reducing steric crowding around the reaction center.
-
Reactions requiring exceptionally good leaving groups: Some reactions necessitate exceptionally good leaving groups, such as those involving very weak nucleophiles or highly stable carbocations. In these cases, Cl⁻ might be too poor a leaving group to drive the reaction efficiently. Sulfonate esters (tosylates, mesylates, triflates) are often preferred in these situations because they are significantly better leaving groups due to resonance stabilization.
Comparing Cl⁻ to other common leaving groups:
| Leaving Group | Conjugate Acid pKa | Relative Leaving Group Ability | Notes |
|---|---|---|---|
| Triflate (OTf⁻) | -14 | Excellent | Highly resonance-stabilized |
| Tosylate (OTs⁻) | -6 | Excellent | Resonance-stabilized |
| Mesylate (OMs⁻) | -2 | Good | Resonance-stabilized |
| Iodide (I⁻) | -10 | Excellent | Large size, high polarizability |
| Bromide (Br⁻) | -9 | Good | |
| Chloride (Cl⁻) | -7 | Good | |
| Water (H₂O) | 15.7 | Poor | |
| Hydroxide (OH⁻) | 15.7 | Very Poor | |
| Acetate (CH₃COO⁻) | 4.76 | Poor |
This table highlights the relative leaving group ability of Cl⁻ in comparison to other common leaving groups. The pKa of the conjugate acid is inversely proportional to the leaving group ability; a lower pKa indicates a stronger acid and a better leaving group.
Factors influencing Cl⁻'s leaving group ability in specific reactions:
The effectiveness of Cl⁻ as a leaving group is profoundly affected by various factors within the reaction context:
-
Solvent: Polar aprotic solvents often enhance the reactivity of SN2 reactions by stabilizing the transition state and increasing the nucleophile's reactivity. However, the impact on Cl⁻'s leaving group ability is less pronounced compared to its effect on the nucleophile.
-
Substrate: The nature of the substrate (alkyl halide) significantly influences the reaction rate. Tertiary alkyl halides react faster in SN1 reactions due to the increased stability of the tertiary carbocation. Primary alkyl halides favour SN2 reactions.
-
Nucleophile: A strong nucleophile facilitates SN2 reactions. The strength of the nucleophile directly influences the reaction rate, but it doesn't directly affect the leaving group ability of Cl⁻.
-
Temperature and pressure: Higher temperatures generally accelerate reaction rates, impacting both the nucleophilic attack and the departure of the leaving group.
-
Presence of catalysts: Catalysts can influence the reaction mechanism and rate by lowering the activation energy, potentially impacting the effectiveness of Cl⁻ as a leaving group in specific situations.
Conclusion: Context is Key
In summary, while chloride ion (Cl⁻) is generally considered a good leaving group in organic chemistry, its performance is intricately linked to the reaction conditions and the nature of the substrate and other reagents. It's not the best leaving group available, often outperformed by other halides (I⁻, Br⁻) and especially sulfonate esters. However, its availability, relatively good stability, and reasonable reactivity make it a frequently used and perfectly acceptable leaving group in many common reactions. Understanding the factors influencing its leaving group ability allows chemists to effectively predict and optimize reaction outcomes. A nuanced approach, considering the entire reaction system, is crucial to accurately assess its effectiveness in any given scenario. Remember that the context of the reaction is paramount in determining whether Cl⁻ is a suitable leaving group.
Latest Posts
Latest Posts
-
What Is A Mixed Melting Point
Mar 20, 2025
-
How Do Hydrogen Ions Flow Through Atp Synthase
Mar 20, 2025
-
How To Draw A 3d Vector
Mar 20, 2025
-
What Is It Called When A Volcano Collapses On Itself
Mar 20, 2025
-
Difference Between Reference And Thematic Maps
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Cl A Good Leaving Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
