How Do Hydrogen Ions Flow Through Atp Synthase
Muz Play
Mar 20, 2025 · 6 min read
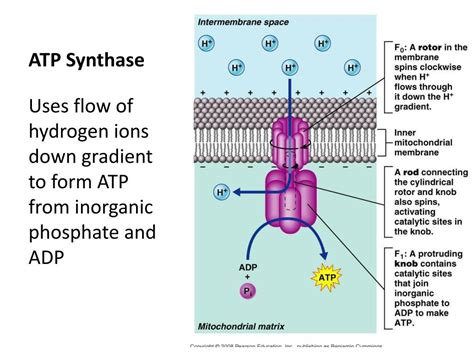
Table of Contents
How Do Hydrogen Ions Flow Through ATP Synthase? Unraveling the Mechanism of Energy Conversion
ATP synthase, a remarkable molecular machine, sits at the heart of cellular energy production. This enzyme is responsible for synthesizing adenosine triphosphate (ATP), the cell's primary energy currency, using the energy stored in a proton gradient. Understanding how hydrogen ions (protons, H+) flow through ATP synthase is crucial to grasping the intricacies of cellular respiration and photosynthesis. This article will delve into the complex mechanism, exploring the structure of ATP synthase, the chemiosmotic theory, and the rotational catalysis model that explains the remarkable efficiency of this molecular marvel.
The Architecture of ATP Synthase: A Molecular Turbine
ATP synthase is a remarkable example of biological nanotechnology. It's a large, multi-subunit enzyme complex embedded in the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes). Its structure can be broadly divided into two main functional domains:
1. F<sub>0</sub> (Fo): The Proton Channel
The F<sub>0</sub> domain is an integral membrane protein complex that acts as a proton channel. This component is crucial for the passage of protons across the membrane. Its structure comprises multiple subunits, most notably the a, b, and c subunits. The c-subunits arrange themselves in a ring, a crucial element in the rotational catalysis model. The a-subunit forms a half-channel, allowing protons to access the c-ring, and the b-subunits act as a stator, anchoring the F<sub>0</sub> domain to the F<sub>1</sub> domain. The precise number of c-subunits varies between organisms, influencing the efficiency of ATP synthesis.
2. F<sub>1</sub> (F1): The Catalytic Core
The F<sub>1</sub> domain is the catalytic core of ATP synthase, protruding into the mitochondrial matrix (or cytoplasm). This domain is responsible for the actual synthesis of ATP. It’s composed of five different subunits: α<sub>3</sub>, β<sub>3</sub>, γ, δ, and ε. The α and β subunits arrange themselves alternately in a hexameric structure. Importantly, the β subunits are the catalytic sites where ADP and inorganic phosphate (Pi) are combined to form ATP. The γ, δ, and ε subunits form a central stalk that connects the F<sub>1</sub> and F<sub>0</sub> domains, acting as a rotor during the process.
The Chemiosmotic Theory: The Driving Force Behind ATP Synthesis
The movement of protons through ATP synthase is driven by the chemiosmotic theory, proposed by Peter Mitchell. This theory explains how the energy stored in an electrochemical proton gradient across a membrane is harnessed to drive ATP synthesis. The gradient is established during electron transport in cellular respiration or the light-dependent reactions of photosynthesis.
This gradient has two components:
- Proton concentration gradient: A higher concentration of protons exists on one side of the membrane (e.g., the intermembrane space in mitochondria) compared to the other (e.g., the mitochondrial matrix).
- Electrical potential gradient: The uneven distribution of protons also creates an electrical potential difference across the membrane due to the positive charge of the protons.
The combined effect of these gradients creates a proton-motive force, which provides the energy to drive protons through the F<sub>0</sub> domain of ATP synthase.
Rotational Catalysis: The Mechanism of ATP Synthesis
The current accepted model for ATP synthesis is the rotational catalysis model. This model elegantly explains how the flow of protons through F<sub>0</sub> drives the rotation of the γ subunit, leading to conformational changes in the β subunits and ultimately ATP synthesis.
Here’s a step-by-step breakdown:
-
Proton Binding: Protons from the high-proton concentration side bind to a specific aspartic acid residue on a c-subunit within the c-ring of F<sub>0</sub>.
-
Rotation of the c-ring: The binding of the proton causes a conformational change in the c-subunit, leading to a rotation of the entire c-ring. This rotation is driven by the proton gradient, with each proton moving the ring a small increment. This is a crucial step where the energy from the proton gradient is directly converted into mechanical rotation.
-
Rotation of the γ subunit: The c-ring is physically connected to the γ subunit through the a-subunit, thus the rotation of the c-ring causes the γ subunit to rotate within the α<sub>3</sub>β<sub>3</sub> hexamer. This rotation acts as a mechanical driver for ATP synthesis.
-
Conformational Changes in β subunits: As the γ subunit rotates, it interacts with each of the three β subunits, causing cyclical conformational changes. These changes are essential for the catalytic cycle. The three β subunits cycle through three distinct conformations:
- Open (O): This conformation has low affinity for nucleotides, allowing ADP and Pi to bind or ATP to be released.
- Loose (L): This conformation binds ADP and Pi loosely.
- Tight (T): This conformation binds ADP and Pi tightly, facilitating the formation of ATP.
-
ATP Synthesis: As the γ subunit rotates, it forces each β subunit to sequentially undergo the O → L → T → O conformational changes. The high-energy conformation (T) facilitates the synthesis of ATP from ADP and Pi. Once formed, ATP is released in the O conformation.
Factors Affecting ATP Synthase Activity
Several factors can influence the efficiency and rate of ATP synthesis by ATP synthase:
- Proton motive force: The strength of the proton gradient directly affects the rate of proton flow and hence ATP synthesis. A stronger gradient leads to faster rotation and higher ATP production.
- pH difference: The pH difference across the membrane is directly related to the proton concentration gradient and thus impacts ATP synthesis.
- Temperature: Temperature influences the enzyme's kinetics, affecting the rate of conformational changes and ATP synthesis.
- Inhibitors: Certain molecules can inhibit ATP synthase activity, disrupting the process at different stages. Examples include oligomycin, which binds to the F<sub>0</sub> subunit, blocking proton flow.
- Uncouplers: Uncouplers dissipate the proton gradient without producing ATP, reducing the efficiency of ATP synthase. Examples include dinitrophenol (DNP).
The Significance of ATP Synthase in Cellular Life
ATP synthase is a vital enzyme in all living organisms. Its role in ATP production fuels a vast array of cellular processes, including:
- Muscle contraction: ATP provides the energy for muscle fiber shortening.
- Active transport: ATP powers the movement of molecules against their concentration gradients.
- Biosynthesis: ATP provides the energy for synthesizing macromolecules like proteins, nucleic acids, and polysaccharides.
- Signal transduction: ATP plays a role in cellular signaling pathways.
- Cellular motility: ATP fuels various forms of cellular movement, such as cilia and flagella beating.
Conclusion: A Molecular Masterpiece
ATP synthase stands as a testament to the elegance and efficiency of biological systems. Its intricate structure and rotational catalysis mechanism allow for the precise conversion of electrochemical energy into chemical energy in the form of ATP. Understanding the flow of hydrogen ions through ATP synthase provides critical insights into the fundamental processes of life and has profound implications for biomedical research and the development of new technologies. Further research continues to unveil the subtle details of this molecular machine, revealing its remarkable adaptability and precision in maintaining cellular energy homeostasis. The continued study of ATP synthase promises further discoveries and advancements in our understanding of cellular energy production and its crucial role in life.
Latest Posts
Latest Posts
-
Responsibilities Of Citizens Of The United States
Mar 20, 2025
-
What Is The Polymer Of A Protein
Mar 20, 2025
-
Difference Between Sn1 And Sn2 Reactions
Mar 20, 2025
-
What Classification Of Alcohol Is Resistant To Oxidation
Mar 20, 2025
-
Is Coffee An Element Compound Or Mixture
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Do Hydrogen Ions Flow Through Atp Synthase . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
