Difference Between Sn1 And Sn2 Reactions
Muz Play
Mar 20, 2025 · 7 min read
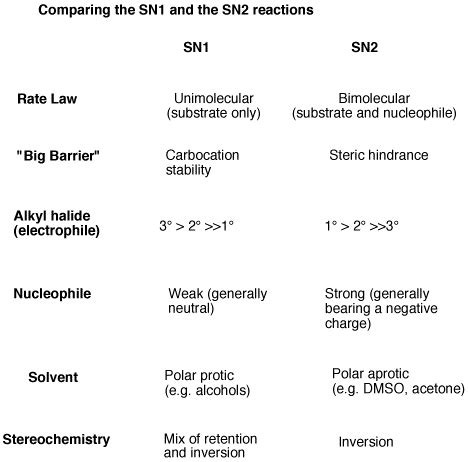
Table of Contents
Unveiling the Differences: SN1 vs. SN2 Reactions
Organic chemistry often feels like navigating a complex maze, and understanding nucleophilic substitution reactions (SN reactions) is a crucial step in mastering this field. Two prominent mechanisms govern these reactions: SN1 and SN2. While both involve the substitution of a leaving group by a nucleophile, their pathways, reaction rates, stereochemistry, and preferred conditions differ significantly. This comprehensive guide will dissect the nuances of SN1 and SN2 reactions, equipping you with the knowledge to confidently predict reaction outcomes.
Understanding the Fundamentals: Nucleophiles and Leaving Groups
Before diving into the mechanisms, let's solidify our understanding of the key players: nucleophiles and leaving groups.
Nucleophiles: The Electron Donors
Nucleophiles are electron-rich species that are attracted to electron-deficient centers. They possess a lone pair of electrons or a π bond that can be donated to form a new bond. Strong nucleophiles are more reactive and readily donate their electrons. The strength of a nucleophile depends on several factors, including its charge (negatively charged nucleophiles are generally stronger), electronegativity (less electronegative atoms are better nucleophiles), and steric hindrance (bulky nucleophiles are less reactive). Examples include:
- Anionic nucleophiles: OH⁻, CN⁻, I⁻, Br⁻, Cl⁻, etc.
- Neutral nucleophiles: H₂O, NH₃, ROH, etc.
Leaving Groups: The Electron Acceptors
Leaving groups are atoms or groups that depart from the substrate, taking with them a pair of electrons. Good leaving groups are stable, weakly basic species that can readily accommodate the negative charge after leaving. The stability of the leaving group is crucial; weaker bases make better leaving groups. Examples include:
- Halides: I⁻, Br⁻, Cl⁻ (I⁻ being the best)
- Tosylate (OTs): A common leaving group derived from tosylic acid.
- Mesylate (OMs): Another excellent leaving group derived from methanesulfonic acid.
SN1 Reactions: A Two-Step Mechanism
SN1 reactions, or unimolecular nucleophilic substitution reactions, proceed through a two-step mechanism involving a carbocation intermediate.
Step 1: Ionization – Formation of the Carbocation
The reaction begins with the ionization of the substrate. The bond between the carbon atom bearing the leaving group and the leaving group breaks heterolytically, meaning the bond electrons are completely transferred to the leaving group. This generates a carbocation intermediate and a free leaving group. The rate of this step is solely determined by the concentration of the substrate. This is why SN1 reactions are first-order reactions; the rate is directly proportional to the concentration of the alkyl halide.
Rate = k[Substrate]
The stability of the carbocation intermediate plays a crucial role. Tertiary carbocations are most stable due to hyperconjugation and inductive effects, followed by secondary and then primary carbocations. Methyl carbocations are extremely unstable and rarely formed. Therefore, SN1 reactions are favored by tertiary substrates.
Step 2: Nucleophilic Attack
Once the carbocation intermediate is formed, the nucleophile attacks the positively charged carbon atom. This step is fast and involves the formation of a new bond between the nucleophile and the carbon. The nucleophile can attack from either side of the planar carbocation, leading to a racemic mixture of products.
SN2 Reactions: A Concerted Mechanism
SN2 reactions, or bimolecular nucleophilic substitution reactions, proceed through a concerted mechanism, meaning the bond breaking and bond formation occur simultaneously in a single step.
The Concerted Step: Backside Attack
The nucleophile attacks the carbon atom bearing the leaving group from the backside, opposite to the leaving group. This backside attack leads to a transition state where the nucleophile and the leaving group are partially bonded to the carbon atom. The transition state is high in energy, and the energy barrier determines the rate of the reaction. Simultaneously, the bond between the carbon and the leaving group breaks, resulting in the substitution of the leaving group by the nucleophile.
Because both the nucleophile and the substrate are involved in the rate-determining step, SN2 reactions are second-order reactions. The rate depends on the concentration of both the substrate and the nucleophile.
Rate = k[Substrate][Nucleophile]
The steric hindrance around the carbon atom bearing the leaving group significantly influences the rate of SN2 reactions. Sterically hindered substrates hinder the backside attack by the nucleophile, slowing down the reaction. Therefore, SN2 reactions are favored by primary substrates, and methyl substrates are the most reactive. Secondary substrates can also undergo SN2 reactions, but the rate is significantly slower compared to primary substrates. Tertiary substrates generally do not undergo SN2 reactions due to excessive steric hindrance.
Comparing SN1 and SN2 Reactions: A Head-to-Head Analysis
| Feature | SN1 Reaction | SN2 Reaction |
|---|---|---|
| Mechanism | Two-step, carbocation intermediate | Concerted, one-step |
| Rate Law | First-order: Rate = k[Substrate] | Second-order: Rate = k[Substrate][Nucleophile] |
| Substrate | Tertiary > Secondary > Primary (Methyl very slow) | Methyl > Primary > Secondary (Tertiary very slow) |
| Nucleophile | Weak or strong nucleophiles can be used | Strong nucleophiles are preferred |
| Leaving Group | Good leaving group is essential | Good leaving group is essential |
| Stereochemistry | Racemization (unless chiral center is hindered) | Inversion of configuration (Walden inversion) |
| Solvent | Polar protic solvents (stabilize carbocation) | Polar aprotic solvents (stabilize nucleophile) |
Factors Affecting SN1 and SN2 Reaction Rates
Several factors influence the rate of both SN1 and SN2 reactions. Understanding these factors is crucial in predicting reaction outcomes and designing synthetic strategies.
Substrate Structure
As discussed, the structure of the substrate significantly affects the reaction pathway. Tertiary substrates favor SN1 reactions due to the stability of the resulting carbocation, while primary substrates favor SN2 reactions due to reduced steric hindrance. Secondary substrates can undergo both SN1 and SN2 reactions, with the dominant pathway depending on the specific reaction conditions and the nature of the nucleophile and solvent.
Nucleophile Strength and Concentration
Strong nucleophiles favor SN2 reactions due to their ability to attack the substrate rapidly. Weak nucleophiles are more likely to participate in SN1 reactions, where the rate-determining step is the formation of the carbocation, and the nucleophile's role is less critical. The concentration of the nucleophile also plays a role; higher concentrations favor SN2 reactions.
Leaving Group Ability
The ability of the leaving group to depart is crucial for both SN1 and SN2 reactions. Good leaving groups, such as halides (I⁻ > Br⁻ > Cl⁻), tosylate, and mesylate, are preferred because they stabilize the negative charge acquired after leaving the substrate. Poor leaving groups, such as hydroxide (OH⁻) and alkoxide (RO⁻), hinder both reaction pathways.
Solvent Effects
The choice of solvent significantly impacts the reaction rate and pathway. Polar protic solvents (e.g., water, alcohols) stabilize the carbocation intermediate in SN1 reactions, increasing their rate. Polar aprotic solvents (e.g., acetone, DMF, DMSO) stabilize the nucleophile in SN2 reactions, enhancing their reactivity.
Applications of SN1 and SN2 Reactions in Organic Synthesis
SN1 and SN2 reactions are fundamental transformations in organic chemistry, serving as building blocks for synthesizing a wide range of molecules. Their mechanistic differences allow chemists to selectively manipulate molecular structures and create desired products. Specific applications include:
- Alkyl halide synthesis: SN2 reactions are commonly used to prepare alkyl halides from alcohols using appropriate reagents.
- Alcohol synthesis: SN1 and SN2 reactions can be used to synthesize alcohols from alkyl halides by reacting them with nucleophilic oxygen species.
- Ether synthesis: SN2 reactions are widely employed in ether synthesis via Williamson ether synthesis.
- Nitrile synthesis: SN2 reactions are used to synthesize nitriles from alkyl halides using cyanide ion (CN⁻) as a nucleophile.
- Ester synthesis: SN2 reactions can be employed in the synthesis of esters.
Conclusion: Mastering the Nuances of SN1 and SN2 Reactions
Understanding the intricate differences between SN1 and SN2 reactions is paramount for success in organic chemistry. By considering factors such as substrate structure, nucleophile strength, leaving group ability, and solvent effects, we can predict reaction outcomes and design efficient synthetic strategies. The ability to differentiate between these mechanisms allows for precise control over reaction pathways, ultimately leading to the synthesis of complex molecules with desired stereochemistry and functional groups. This detailed comparison provides a robust foundation for tackling more advanced organic chemistry concepts and further exploring the fascinating world of nucleophilic substitution.
Latest Posts
Latest Posts
-
The Long Love That In My Thought Doth Harbor
Mar 21, 2025
-
Function Of The Stage Of A Microscope
Mar 21, 2025
-
Periodic Table Solids Liquids And Gases
Mar 21, 2025
-
Coefficient Of Performance For Refrigeration Cycle
Mar 21, 2025
-
How To Calculate Moles From Volume
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Sn1 And Sn2 Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
