How To Calculate Moles From Volume
Muz Play
Mar 21, 2025 · 5 min read
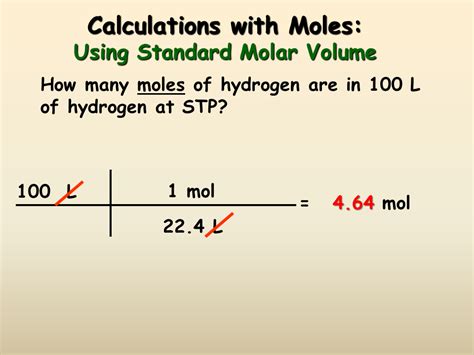
Table of Contents
How to Calculate Moles from Volume: A Comprehensive Guide
Calculating the number of moles from volume is a fundamental concept in chemistry, crucial for various applications, from stoichiometric calculations to determining solution concentrations. Understanding this process requires a grasp of molar volume, density, and the ideal gas law, depending on the state of the substance. This comprehensive guide will walk you through different scenarios and methods to accurately calculate moles from volume.
Understanding Moles and Volume
Before diving into the calculations, let's clarify the key terms.
Moles (mol): A mole represents a specific number of particles (atoms, molecules, ions, etc.), defined as Avogadro's number (approximately 6.022 x 10²³). It's a fundamental unit in chemistry for measuring the amount of substance.
Volume (L or mL): Volume refers to the amount of three-dimensional space occupied by a substance. It's usually expressed in liters (L) or milliliters (mL) in chemistry.
The relationship between moles and volume depends on the physical state of the substance: solid, liquid, or gas.
Calculating Moles from Volume: Liquids and Solids
For liquids and solids, the key to calculating moles from volume is density. Density (ρ) is defined as mass (m) per unit volume (V):
ρ = m/V
We can use this relationship, along with the molar mass (M), to determine the number of moles (n):
n = m/M
Combining these equations, we get:
n = (ρV)/M
Where:
- n = number of moles
- ρ = density (g/mL or g/L)
- V = volume (mL or L)
- M = molar mass (g/mol)
Example 1: Calculating Moles of Ethanol
Let's say we have 250 mL of ethanol (C₂H₅OH) with a density of 0.789 g/mL. The molar mass of ethanol is 46.07 g/mol. How many moles of ethanol are present?
- Convert volume to liters (if necessary): 250 mL = 0.250 L
- Use the formula: n = (ρV)/M = (0.789 g/mL * 250 mL) / 46.07 g/mol
- Calculate: n ≈ 4.27 moles
Important Considerations for Liquids and Solids:
- Accuracy of Density: The accuracy of your mole calculation directly depends on the accuracy of the density value. Ensure you're using a reliable source for density data and consider the temperature, as density is temperature-dependent.
- Purity of Substance: The calculation assumes the substance is pure. Impurities will affect the density and thus the mole calculation.
Calculating Moles from Volume: Gases
Calculating moles from the volume of a gas is different from liquids and solids because gas volume is highly sensitive to temperature and pressure. The ideal gas law provides the necessary relationship:
PV = nRT
Where:
- P = pressure (usually in atmospheres, atm)
- V = volume (usually in liters, L)
- n = number of moles
- R = ideal gas constant (0.0821 L·atm/mol·K)
- T = temperature (in Kelvin, K)
To calculate moles (n), rearrange the ideal gas law:
n = PV/RT
Example 2: Calculating Moles of Oxygen Gas
Suppose we have 5.0 L of oxygen gas (O₂) at a pressure of 1.2 atm and a temperature of 298 K. How many moles of oxygen gas are present?
- Use the formula: n = PV/RT = (1.2 atm * 5.0 L) / (0.0821 L·atm/mol·K * 298 K)
- Calculate: n ≈ 0.245 moles
Important Considerations for Gases:
- Ideal Gas Law Assumptions: The ideal gas law is an approximation. Real gases deviate from ideal behavior, especially at high pressures and low temperatures. For more accurate calculations under non-ideal conditions, you might need to use more complex equations of state.
- Units: Maintaining consistent units is crucial. If you use different units for pressure, volume, or temperature, your calculation will be incorrect.
- Partial Pressures: If the gas is a mixture, you'll need to use the partial pressure of the specific gas you're interested in. Dalton's law of partial pressures states that the total pressure is the sum of the partial pressures of each gas in the mixture.
Calculating Moles from Volume: Solutions
For solutions, the concentration is typically expressed in molarity (M), which is defined as moles of solute per liter of solution:
Molarity (M) = moles of solute (n) / volume of solution (V)
Therefore, to find the number of moles, rearrange the equation:
n = M * V
Example 3: Calculating Moles of Sodium Chloride in a Solution
You have 250 mL of a 0.5 M sodium chloride (NaCl) solution. How many moles of NaCl are present?
- Convert volume to liters: 250 mL = 0.250 L
- Use the formula: n = M * V = 0.5 mol/L * 0.250 L
- Calculate: n = 0.125 moles
Important Considerations for Solutions:
- Accurate Measurement of Volume: Precise volume measurement is critical for accurate mole calculations. Use appropriate volumetric glassware, such as volumetric flasks and pipettes.
- Solution Preparation: If you're preparing the solution yourself, ensure it's properly mixed and homogeneous before taking volume measurements.
Advanced Techniques and Applications
The methods described above provide the foundation for calculating moles from volume. However, more advanced techniques are needed in specific scenarios:
- Titration: Titration is a common laboratory technique to determine the concentration of a solution by reacting it with a solution of known concentration. The stoichiometry of the reaction is crucial for calculating the moles of the unknown substance.
- Gas Chromatography: Gas chromatography separates and quantifies the components of a gas mixture. The area under each peak is proportional to the amount of the substance, which can then be converted to moles.
- Spectroscopy: Various spectroscopic techniques (UV-Vis, IR, NMR) can be used to determine the concentration of a substance, which can then be converted to moles using the appropriate molar absorptivity or other relevant parameters.
Conclusion
Calculating moles from volume is a critical skill in chemistry, with applications in various fields. The approach depends on the state of the matter (solid, liquid, or gas) and whether the substance is part of a solution. While the fundamental principles are relatively straightforward, accurate measurements and a thorough understanding of the relevant equations and concepts are crucial for obtaining reliable results. Remember to always pay close attention to units and consider potential sources of error. This guide provides a solid foundation for mastering these calculations and applying them effectively in your chemical studies or work.
Latest Posts
Latest Posts
-
Atom Equivalent To 7 Atoms Of Hydrogen
Mar 27, 2025
-
Evaluate The Integral By Changing To Spherical Coordinates
Mar 27, 2025
-
Are Double Bonds Stronger Than Single Bonds
Mar 27, 2025
-
Consumer Surplus With A Price Floor
Mar 27, 2025
-
Ground State Electron Configuration Of C
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Moles From Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
