What Is The Difference Between Ionic And Molecular
Muz Play
Mar 24, 2025 · 6 min read
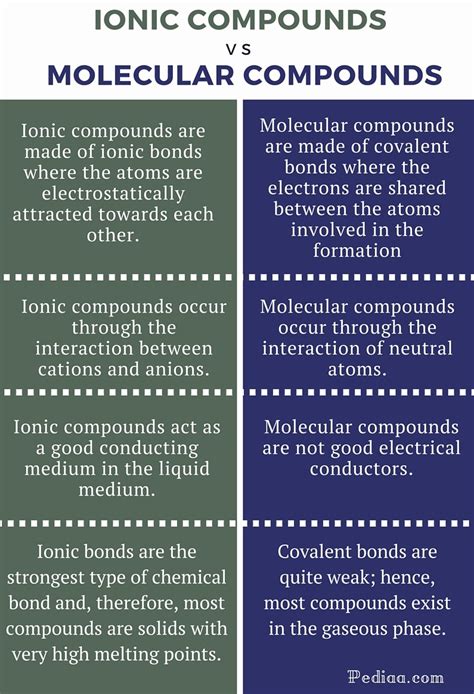
Table of Contents
Delving Deep: The Differences Between Ionic and Molecular Compounds
Understanding the fundamental differences between ionic and molecular compounds is crucial for grasping core concepts in chemistry. These differences extend beyond simple definitions, influencing their physical properties, chemical behavior, and applications in various fields. This comprehensive guide will explore these differences in detail, providing a thorough understanding suitable for both beginners and those seeking a deeper dive into chemical bonding.
The Building Blocks: Atoms, Ions, and Molecules
Before diving into the distinctions between ionic and molecular compounds, let's establish a clear understanding of the basic building blocks: atoms, ions, and molecules.
Atoms: The Fundamental Units
Atoms are the basic units of matter, composed of a nucleus containing protons and neutrons, surrounded by orbiting electrons. The number of protons determines the element's identity (e.g., hydrogen has one proton, oxygen has eight). Atoms are electrically neutral, possessing an equal number of protons and electrons.
Ions: Charged Atoms
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge. A cation is a positively charged ion, formed when an atom loses one or more electrons. Conversely, an anion is a negatively charged ion, formed when an atom gains one or more electrons. The formation of ions is a crucial step in the creation of ionic compounds.
Molecules: Bound Atoms
A molecule is formed when two or more atoms are chemically bonded together. These bonds can be covalent (sharing electrons) or coordinate covalent (one atom providing both electrons for the bond). Molecules can be composed of atoms of the same element (e.g., O₂, oxygen gas) or different elements (e.g., H₂O, water).
Ionic Compounds: The Electrostatic Attraction
Ionic compounds are formed through the electrostatic attraction between oppositely charged ions (cations and anions). This attraction arises from the transfer of electrons from one atom to another, creating ions with significantly different electronegativities. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond.
Formation of Ionic Bonds
The formation of an ionic bond typically involves a metal (which tends to lose electrons easily) and a nonmetal (which tends to gain electrons easily). For instance, in the formation of sodium chloride (NaCl, table salt), sodium (Na), a metal, loses one electron to become a sodium cation (Na⁺), while chlorine (Cl), a nonmetal, gains that electron to become a chloride anion (Cl⁻). The strong electrostatic attraction between the positively charged sodium ion and the negatively charged chloride ion forms the ionic bond.
Properties of Ionic Compounds
Ionic compounds exhibit several characteristic properties:
- High melting and boiling points: The strong electrostatic forces between ions require significant energy to overcome, resulting in high melting and boiling points.
- Crystalline structure: Ionic compounds typically form a regular, crystalline structure due to the ordered arrangement of ions to maximize electrostatic attraction and minimize repulsion.
- Solubility in polar solvents: Ionic compounds often dissolve readily in polar solvents like water, which can disrupt the electrostatic interactions between ions.
- Electrical conductivity: When molten or dissolved in water, ionic compounds conduct electricity because the ions are free to move and carry charge.
- Brittleness: Ionic crystals are brittle because the displacement of ions can lead to repulsion between like charges, causing the crystal to fracture.
Molecular Compounds: The Sharing is Caring Approach
Molecular compounds, also known as covalent compounds, are formed through the sharing of electrons between atoms. This sharing creates covalent bonds, where the atoms are held together by the mutual attraction to the shared electrons.
Formation of Covalent Bonds
Covalent bonds typically occur between nonmetals, which have similar electronegativities. Instead of transferring electrons, the atoms share electrons to achieve a stable electron configuration, usually resembling a noble gas. For example, in water (H₂O), each hydrogen atom shares one electron with the oxygen atom, and the oxygen atom shares one electron with each hydrogen atom, resulting in two covalent bonds.
Types of Covalent Bonds
Several types of covalent bonds exist, including:
- Single bonds: Involve the sharing of one pair of electrons.
- Double bonds: Involve the sharing of two pairs of electrons.
- Triple bonds: Involve the sharing of three pairs of electrons.
The bond strength increases with the number of shared electron pairs.
Properties of Molecular Compounds
Molecular compounds exhibit distinct properties compared to ionic compounds:
- Lower melting and boiling points: The weaker intermolecular forces (e.g., van der Waals forces, hydrogen bonds) between molecules require less energy to overcome, leading to lower melting and boiling points compared to ionic compounds.
- Variable solubility: Solubility varies greatly depending on the polarity of the molecule and the solvent. Polar molecules tend to dissolve in polar solvents, while nonpolar molecules dissolve in nonpolar solvents.
- Poor electrical conductivity: Molecular compounds generally do not conduct electricity because they do not have free-moving ions. Exceptions exist for some substances that ionize in solution.
- Varied physical states: Molecular compounds can exist as solids, liquids, or gases at room temperature depending on their intermolecular forces and molecular weight.
Key Differences Summarized: A Table for Clarity
The following table summarizes the key differences between ionic and molecular compounds:
| Feature | Ionic Compounds | Molecular Compounds |
|---|---|---|
| Bonding | Electrostatic attraction between ions | Sharing of electrons between atoms |
| Formation | Metal + Nonmetal | Nonmetal + Nonmetal |
| Melting Point | High | Low |
| Boiling Point | High | Low |
| Solubility | Often soluble in polar solvents | Varies depending on polarity |
| Electrical Conductivity | Conducts when molten or dissolved in water | Generally does not conduct electricity |
| Structure | Crystalline | Variable, often less structured than ionic |
| Hardness | Brittle | Can vary greatly |
| Examples | NaCl (table salt), MgO (magnesium oxide) | H₂O (water), CO₂ (carbon dioxide), CH₄ (methane) |
Beyond the Basics: Exploring More Complex Scenarios
While the above distinctions provide a solid foundation, it's important to acknowledge that some compounds exhibit characteristics of both ionic and molecular compounds. These cases often involve polar covalent bonds, where the electron sharing is unequal, resulting in a partial positive and partial negative charge within the molecule. This partial charge can lead to stronger intermolecular forces and some ionic-like properties.
Furthermore, the concept of "percent ionic character" helps to quantify the degree of ionic character in a bond. This is dependent on the electronegativity difference between the atoms involved in the bond. A large electronegativity difference suggests a high degree of ionic character, while a small difference indicates a predominantly covalent bond.
Conclusion: A Foundation for Further Exploration
Understanding the differences between ionic and molecular compounds is fundamental to comprehending chemical reactions, predicting properties of substances, and interpreting experimental data. While this guide provides a comprehensive overview, it serves as a stepping stone for more in-depth exploration of chemical bonding, reactivity, and the fascinating world of chemistry. The more you delve into the specifics of each type of compound, the more you will appreciate the elegance and complexity of the interactions that govern our physical world. Remember that chemistry is a dynamic field with continuous discoveries and refinements, so always remain curious and keep exploring!
Latest Posts
Latest Posts
-
As A Balloon Is Inflated What Happens To The Pressure
Mar 30, 2025
-
How To Find The Heat Capacity Of A Calorimeter
Mar 30, 2025
-
Define The Simplest Form Of A Rate
Mar 30, 2025
-
How To Add Radicals With Different Radicands
Mar 30, 2025
-
The Ground State In An Atom Is
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Ionic And Molecular . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
