What Is The Difference Between Products And Reactants
Muz Play
Mar 23, 2025 · 5 min read
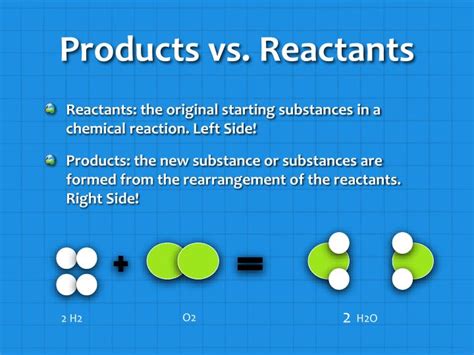
Table of Contents
What's the Difference Between Products and Reactants? A Deep Dive into Chemical Reactions
Understanding the difference between products and reactants is fundamental to grasping the core concepts of chemistry. While seemingly simple, this distinction underpins our comprehension of chemical reactions, their mechanisms, and their applications in various fields. This article will delve deep into the definitions, characteristics, and crucial distinctions between products and reactants, providing a comprehensive understanding for students and enthusiasts alike.
Defining Reactants and Products: The Heart of Chemical Reactions
At the heart of any chemical reaction lies a transformation of substances. We start with reactants, which are the initial substances that undergo a chemical change. These reactants interact with each other, breaking and forming bonds to create entirely new substances known as products. Think of it like baking a cake: your reactants are the flour, sugar, eggs, and butter, while the delicious cake is your product.
Reactants: These are the starting materials in a chemical reaction. They are consumed during the reaction, meaning their amounts decrease as the reaction progresses. Their properties are altered fundamentally during the reaction, resulting in the formation of products with entirely different characteristics.
Products: These are the substances formed as a result of a chemical reaction. They are the end result of the rearrangement of atoms and molecules from the reactants. Products possess distinct physical and chemical properties that differ from the original reactants.
Visualizing the Transformation: Chemical Equations
Chemical equations serve as a concise and powerful tool for representing chemical reactions. They depict the transformation of reactants into products using chemical formulas and stoichiometric coefficients. For example, consider the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation:
- CH₄ (Methane) and O₂ (Oxygen) are the reactants. They are the substances that react with each other.
- CO₂ (Carbon Dioxide) and H₂O (Water) are the products. They are the substances formed as a result of the reaction.
The arrow (→) indicates the direction of the reaction, showing the transformation from reactants to products. The coefficients (numbers in front of the chemical formulas) represent the stoichiometric ratios – the relative amounts of reactants and products involved in the reaction.
Key Differences Summarized: Reactants vs. Products
| Feature | Reactants | Products |
|---|---|---|
| Definition | Starting materials in a chemical reaction | Substances formed in a chemical reaction |
| State | Initially present, consumed during reaction | Formed during the reaction, accumulate over time |
| Properties | Different from the products | Different from the reactants |
| Representation | Appear on the left side of the equation | Appear on the right side of the equation |
| Energy Change | May absorb or release energy | May absorb or release energy (often opposite to reactants) |
Beyond the Basics: Exploring the Nuances
The distinction between reactants and products is not always absolute and straightforward. Several factors can add layers of complexity to this seemingly simple concept:
Reversible Reactions: The Dynamic Equilibrium
Many chemical reactions are reversible, meaning they can proceed in both directions. In such cases, the products can react to reform the reactants. For example:
N₂ + 3H₂ ⇌ 2NH₃
The double arrow (⇌) indicates a reversible reaction. At equilibrium, the rates of the forward (reactants to products) and reverse (products to reactants) reactions are equal. Both reactants and products are present in a dynamic equilibrium.
Reaction Mechanisms and Intermediates
Chemical reactions rarely occur in a single step. They often involve a series of intermediate steps, each with its own reactants and products. These intermediate species are formed during the reaction but are consumed before the final products are formed. Their presence highlights the complex pathways involved in transforming reactants into products.
Catalysts: Accelerating the Transformation
Catalysts are substances that increase the rate of a chemical reaction without being consumed themselves. They participate in the reaction but are not considered either reactants or products because they are regenerated at the end of the reaction. They accelerate the transition from reactants to products by providing an alternative, lower-energy reaction pathway.
Excess Reactants and Limiting Reactants
In many reactions, one reactant is present in excess, while another is the limiting reactant. The limiting reactant determines the amount of product that can be formed. Once the limiting reactant is completely consumed, the reaction stops, even if there is still excess of other reactants.
Side Reactions and Byproducts
In many real-world reactions, multiple reactions occur simultaneously, leading to the formation of unintended byproducts alongside the desired products. Understanding these side reactions is crucial in optimizing reaction conditions to maximize the yield of the desired product and minimize the unwanted byproducts.
Applications Across Disciplines: The Significance of Understanding Reactants and Products
The ability to distinguish and manipulate reactants and products is crucial across diverse scientific and technological disciplines:
- Industrial Chemistry: Optimizing chemical processes to maximize product yield and minimize waste requires a deep understanding of reactants, products, and reaction kinetics.
- Pharmaceutical Chemistry: Developing new drugs involves carefully controlling reactions to synthesize specific products with desired properties and minimize side reactions and byproducts.
- Environmental Science: Understanding chemical reactions in the environment, such as those involved in pollution and remediation, requires a precise understanding of reactants and products.
- Biochemistry: Metabolic processes in living organisms involve complex networks of reactions, where specific reactants are transformed into specific products through enzymatic catalysis.
- Materials Science: Designing and synthesizing new materials with specific properties relies on controlling chemical reactions to produce specific products with tailored characteristics.
Conclusion: Mastering the Fundamentals
The fundamental distinction between reactants and products provides the foundation for comprehending chemical reactions and their applications. By understanding the characteristics, behaviors, and interactions of reactants and products, we unlock the potential to manipulate chemical processes, design new materials, develop new drugs, and address environmental challenges. This understanding is not simply an academic exercise; it is the cornerstone of countless advancements in science and technology, shaping our modern world. While seemingly basic, a firm grasp of this concept allows us to appreciate the complex dynamics of the chemical world and the transformative power of chemical reactions. Further exploration into the intricacies of reaction kinetics, thermodynamics, and mechanisms will only deepen this fundamental understanding, offering even more exciting insights into the world of chemistry.
Latest Posts
Latest Posts
-
A Bond In Which Electrons Are Equally Shared
Mar 24, 2025
-
Maxima And Minima Of Functions Of Three Variables Problems
Mar 24, 2025
-
Issue Of Common Stock Journal Entry
Mar 24, 2025
-
Position Of The Sun Moon And Earth
Mar 24, 2025
-
What Is The Difference Between Supportive And Defensive Communication
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Products And Reactants . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
