A Bond In Which Electrons Are Equally Shared
Muz Play
Mar 24, 2025 · 7 min read
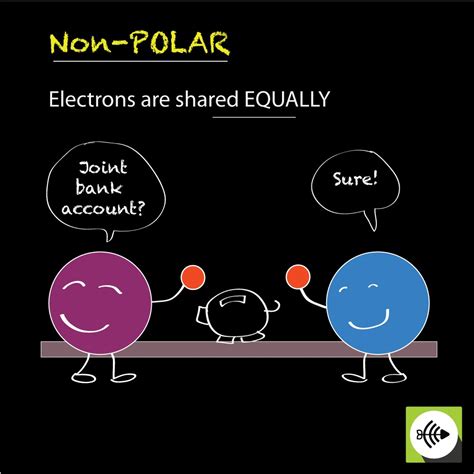
Table of Contents
A Bond in Which Electrons are Equally Shared: Understanding Nonpolar Covalent Bonds
A fundamental concept in chemistry is the chemical bond, the force that holds atoms together to form molecules and compounds. Among the various types of chemical bonds, the covalent bond stands out for its crucial role in the structure and properties of many substances, including the building blocks of life. Within the realm of covalent bonds lies a specific type where electron sharing is perfectly balanced: the nonpolar covalent bond. This article will delve deep into the nature of nonpolar covalent bonds, exploring their characteristics, examples, and significance in the broader context of chemistry.
Defining Nonpolar Covalent Bonds
A covalent bond forms when two atoms share one or more pairs of electrons to achieve a more stable electron configuration, typically resembling that of a noble gas. This sharing occurs because atoms involved often have relatively high electronegativity values, meaning they strongly attract electrons. In a nonpolar covalent bond, the sharing of electrons between the two atoms is equal. This equal sharing arises when the two atoms involved have similar or identical electronegativities. Electronegativity is a measure of an atom's ability to attract shared electrons in a chemical bond.
Consequently, there's no significant difference in the electron density around each atom. The electron cloud is evenly distributed between the atoms, resulting in a zero dipole moment. A dipole moment is a measure of the separation of positive and negative charges within a molecule. In a nonpolar covalent bond, this separation is absent because the electrons are shared equally.
Contrasting Nonpolar and Polar Covalent Bonds
To fully appreciate nonpolar covalent bonds, it's essential to contrast them with their counterparts: polar covalent bonds. In a polar covalent bond, the electrons are shared unequally between the atoms involved. This unequal sharing arises from a difference in electronegativity between the atoms. The atom with the higher electronegativity attracts the shared electrons more strongly, resulting in a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. This creates a dipole moment, making the molecule polar.
For instance, consider the water molecule (H₂O). Oxygen is significantly more electronegative than hydrogen. Consequently, the oxygen atom attracts the shared electrons more strongly, leading to a partial negative charge on the oxygen and partial positive charges on the hydrogens. This polarity is responsible for many of water's unique properties. In contrast, a molecule like oxygen gas (O₂) forms a nonpolar covalent bond because both oxygen atoms have the same electronegativity, resulting in an equal sharing of electrons.
Factors Affecting Nonpolar Covalent Bond Formation
Several factors influence the formation of nonpolar covalent bonds:
1. Electronegativity Difference:
The most crucial factor is the electronegativity difference between the atoms. When the electronegativity difference is close to zero (typically less than 0.4 on the Pauling scale), a nonpolar covalent bond is formed. The smaller the electronegativity difference, the more equal the electron sharing.
2. Atomic Size:
While electronegativity is paramount, the atomic size of the participating atoms can also subtly influence bond polarity. Atoms of similar sizes are more likely to form nonpolar bonds as the electron cloud is less distorted by significant size differences.
3. Bond Length:
The bond length, the distance between the nuclei of two bonded atoms, also plays a minor role. In nonpolar bonds, the bond length tends to be closer to the average of the individual atomic radii than in polar bonds. However, bond length is usually a consequence of the electronegativity difference, rather than a primary determinant.
4. Molecular Symmetry:
For larger molecules with multiple bonds, molecular symmetry can influence the overall polarity. Even if individual bonds are slightly polar, symmetrical arrangement of these bonds can cancel out the dipole moments, resulting in a nonpolar molecule. Carbon tetrachloride (CCl₄) is a classic example.
Examples of Nonpolar Covalent Bonds and Molecules
Several examples showcase nonpolar covalent bonds:
-
Diatomic Molecules: Many diatomic elements, like hydrogen (H₂), oxygen (O₂), nitrogen (N₂), fluorine (F₂), chlorine (Cl₂), bromine (Br₂), and iodine (I₂), form nonpolar covalent bonds due to the identical electronegativities of the atoms within each molecule.
-
Homonuclear Diatomic Molecules: These molecules consist of two atoms of the same element, hence the term "homonuclear." The identical electronegativities guarantee equal electron sharing.
-
Hydrocarbons: Many hydrocarbons, such as methane (CH₄), ethane (C₂H₆), and propane (C₃H₈), consist primarily of carbon-carbon and carbon-hydrogen bonds. The electronegativity difference between carbon and hydrogen is relatively small, resulting in largely nonpolar bonds. However, it's important to note that even in hydrocarbons, slight polarity can exist due to the subtle electronegativity difference.
-
Other Examples: Other examples include molecules like phosphorus (P₄), sulfur (S₈), and many symmetrical organic molecules where the distribution of electronegative atoms results in a net zero dipole moment.
Properties of Substances with Nonpolar Covalent Bonds
Substances formed predominantly by nonpolar covalent bonds exhibit specific properties:
-
Low Melting and Boiling Points: The weak intermolecular forces (London Dispersion Forces) between nonpolar molecules result in low melting and boiling points. These forces are weaker compared to the stronger dipole-dipole or hydrogen bonding found in polar molecules.
-
Poor Solubility in Water: Nonpolar substances generally don't dissolve well in water, a highly polar solvent. The principle of "like dissolves like" applies, meaning polar solvents dissolve polar substances, and nonpolar solvents dissolve nonpolar substances.
-
Good Solubility in Nonpolar Solvents: Nonpolar substances readily dissolve in nonpolar solvents such as hexane or benzene.
-
Non-Conductivity of Electricity: Nonpolar substances generally do not conduct electricity either in solid, liquid, or gaseous states. This is because they lack free-moving charged particles (ions or electrons).
-
Low Surface Tension and Viscosity: These properties are often lower in substances with nonpolar covalent bonds compared to polar ones.
Importance and Applications
Nonpolar covalent bonds are crucial in numerous areas:
-
Organic Chemistry: They form the backbone of organic molecules, including hydrocarbons, fats, oils, and many other biologically important compounds.
-
Biochemistry: Many biological molecules rely on nonpolar interactions for their structure and function. For instance, hydrophobic interactions, driven by the tendency of nonpolar molecules to avoid water, play a vital role in protein folding and membrane structure.
-
Materials Science: Understanding nonpolar bonds is essential in developing new materials with specific properties. For example, polymers with nonpolar components might have specific insulation or hydrophobic properties.
-
Industrial Applications: Nonpolar solvents are extensively used in various industrial processes, including cleaning, extraction, and chemical synthesis.
Advanced Concepts and Exceptions
While the concept of nonpolar covalent bonds is straightforward, some nuances and exceptions deserve consideration:
-
No Perfectly Nonpolar Bonds: Strictly speaking, perfectly nonpolar bonds are rare. Even in homonuclear diatomic molecules, slight differences in the electron environment around each atom can create a tiny dipole moment. However, these moments are often negligible for practical purposes.
-
Influence of Molecular Geometry: The overall polarity of a molecule depends not only on individual bond polarities but also on the molecular geometry. A molecule with polar bonds can be nonpolar if the geometry is symmetrical, causing the individual bond dipoles to cancel each other out.
-
Inductive Effects: In organic chemistry, inductive effects can slightly alter the electron distribution in a bond, even if the electronegativity difference is minimal.
Conclusion
Nonpolar covalent bonds are a fundamental type of chemical bond characterized by the equal sharing of electrons between atoms with similar electronegativities. Understanding these bonds is crucial for comprehending the properties and behavior of a wide range of substances, from simple diatomic molecules to complex biological molecules and advanced materials. While perfectly nonpolar bonds are idealized, the concept provides a valuable framework for understanding the relationship between bonding, molecular structure, and macroscopic properties. The equal sharing of electrons in these bonds contrasts with the unequal sharing in polar covalent bonds, highlighting the diverse ways atoms interact to form stable compounds. Their importance spans across various scientific disciplines, making them a critical concept in chemistry and its related fields.
Latest Posts
Latest Posts
-
How Do You Know If A Reaction Is Redox
Mar 26, 2025
-
Como Sacar El Diametro De Un Circulo
Mar 26, 2025
-
How To Find All Zeros Of A Polynomial
Mar 26, 2025
-
How Do You Calculate The Heat Capacity Of A Calorimeter
Mar 26, 2025
-
Mendels Dihybrid Crosses Supported The Independent Hypothesis
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about A Bond In Which Electrons Are Equally Shared . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
