What Is The Electron Configuration For N
Muz Play
Mar 21, 2025 · 6 min read
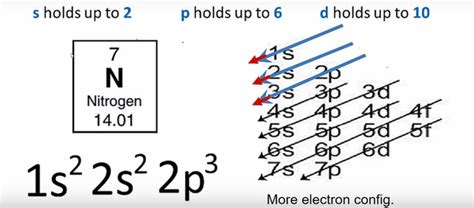
Table of Contents
What is the Electron Configuration for Nitrogen? Understanding Atomic Structure and Orbital Filling
Nitrogen, a crucial element for life as we know it, boasts a fascinating electron configuration that dictates its chemical properties and reactivity. Understanding this configuration requires a grasp of fundamental atomic structure and the principles governing electron orbital filling. This article delves deep into the electron configuration of nitrogen, exploring its implications and relevance in various scientific fields.
Understanding Atomic Structure: The Foundation of Electron Configuration
Before diving into nitrogen's specific electron configuration, let's establish a solid foundation by reviewing the basic principles of atomic structure. Atoms are composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number.
- Neutrons: Neutrally charged particles also located in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. These electrons determine an atom's chemical behavior and are the focus of electron configuration.
Electron Shells and Subshells: Organizing the Electron Cloud
Electrons don't randomly orbit the nucleus; they occupy distinct energy levels known as shells. These shells are further divided into subshells, each characterized by a specific shape and capable of holding a limited number of electrons. The subshells are denoted by the letters s, p, d, and f.
- s subshell: Spherical in shape, holding a maximum of 2 electrons.
- p subshell: Dumbbell-shaped, holding a maximum of 6 electrons (3 orbitals, 2 electrons per orbital).
- d subshell: More complex shapes, holding a maximum of 10 electrons (5 orbitals).
- f subshell: Even more complex shapes, holding a maximum of 14 electrons (7 orbitals).
The filling of these subshells follows specific rules, outlined in the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
The Aufbau Principle: Building Up the Electron Configuration
The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy. This means that lower energy levels are filled before higher energy levels. The order of filling is generally as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
However, there are exceptions to this rule, particularly with transition metals and some heavier elements.
Hund's Rule: Maximizing Unpaired Electrons
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This maximizes the total spin of the electrons, leading to greater stability.
The Pauli Exclusion Principle: One Electron Per Orbital Spin State
The Pauli exclusion principle dictates that no two electrons in an atom can have the same four quantum numbers. This means that each orbital can hold a maximum of two electrons, but they must have opposite spins (represented as ↑ and ↓).
Determining the Electron Configuration for Nitrogen (N)
Nitrogen's atomic number is 7, meaning it has 7 protons and, in a neutral atom, 7 electrons. Using the Aufbau principle, Hund's rule, and the Pauli exclusion principle, we can determine its electron configuration:
1s² 2s² 2p³
Let's break this down:
- 1s²: The first shell (n=1) contains the s subshell, which holds 2 electrons.
- 2s²: The second shell (n=2) contains the s subshell, which also holds 2 electrons.
- 2p³: The second shell (n=2) also contains the p subshell, which can hold up to 6 electrons. Nitrogen only has 3 electrons in this subshell, each occupying a separate p orbital with parallel spins, according to Hund's rule.
Therefore, the complete electron configuration for nitrogen is 1s² 2s² 2p³. This configuration is crucial for understanding nitrogen's chemical behavior.
The Significance of Nitrogen's Electron Configuration
Nitrogen's electron configuration has far-reaching implications, impacting its:
Chemical Reactivity:
The three unpaired electrons in the 2p subshell make nitrogen relatively reactive, readily forming covalent bonds with other atoms to achieve a stable octet (eight electrons in its outermost shell). This is why nitrogen forms numerous compounds, including ammonia (NH₃), nitric acid (HNO₃), and various nitrogen oxides.
Bonding Properties:
The presence of three unpaired electrons allows nitrogen to form three single bonds, as seen in ammonia. It can also form multiple bonds, such as the triple bond in nitrogen gas (N₂), where each nitrogen atom shares three electrons with the other. This triple bond is exceptionally strong, accounting for the inertness of nitrogen gas under normal conditions.
Biological Importance:
Nitrogen is an essential component of amino acids, the building blocks of proteins, and nucleic acids, the building blocks of DNA and RNA. Its ability to form stable covalent bonds is fundamental to life's molecular machinery.
Industrial Applications:
Nitrogen's unique properties are exploited in various industrial applications. Nitrogen gas is used as an inert atmosphere in food packaging, preventing oxidation and spoilage. It's also used in the production of ammonia for fertilizers, a cornerstone of modern agriculture. Furthermore, nitrogen compounds find use in explosives and various chemical processes.
Beyond the Basics: Orbital Diagrams and Quantum Numbers
To gain a deeper understanding of nitrogen's electron configuration, it's helpful to visualize it using orbital diagrams and consider quantum numbers.
Orbital Diagrams:
Orbital diagrams provide a visual representation of electron distribution within subshells. For nitrogen, the 2p subshell would be depicted as three separate boxes, each representing a p orbital, with one electron in each box (↑) before pairing starts.
Quantum Numbers:
Each electron in an atom is described by four quantum numbers:
- Principal quantum number (n): Specifies the energy level (shell). For nitrogen's outermost electrons, n=2.
- Azimuthal quantum number (l): Specifies the subshell (s=0, p=1, d=2, f=3). For nitrogen's outermost electrons, l=1 (p subshell).
- Magnetic quantum number (ml): Specifies the orbital within a subshell (-l to +l). For nitrogen's 2p electrons, ml can be -1, 0, or +1.
- Spin quantum number (ms): Specifies the electron's spin (+1/2 or -1/2).
Knowing these quantum numbers allows for a precise description of each electron's location and energy within the atom.
Conclusion: The Significance of Understanding Electron Configuration
Understanding the electron configuration of nitrogen is crucial for comprehending its chemical behavior, bonding properties, and its pivotal role in various scientific disciplines. From the strength of the nitrogen triple bond to its essential role in biological molecules, its electron configuration provides the fundamental explanation for its importance in our world. This knowledge enables scientists and engineers to leverage nitrogen's unique characteristics in diverse fields, from agriculture to materials science. The principles discussed here, including the Aufbau principle, Hund's rule, and the Pauli exclusion principle, are not limited to nitrogen but are applicable to understanding the electron configurations of all elements in the periodic table, laying the foundation for a deeper understanding of chemistry and the behavior of matter. Further exploration of these principles will unlock a wealth of knowledge about the intricate world of atomic structure and chemical bonding.
Latest Posts
Latest Posts
-
What Is The Radius Of Hydrogen
Mar 28, 2025
-
Is Trigonal Planar Polar Or Nonpolar
Mar 28, 2025
-
What Is The Unique Property Of Water
Mar 28, 2025
-
What Is A Particle With A Negative Charge
Mar 28, 2025
-
Why Does Water Have High Heat Of Vaporization
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For N . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
